One of the unanticipated consequences of being a bio-mechanical researcher focused on cartilage health and joint mobility is that people you encounter are often compelled to share their own experiences with chronic joint pain. While potentially exhausting for some, Mechanical Engineering Associate Professor Nic Fiorentino welcomes these exchanges as they fuel his drive to improve human health through scientific investigations of tissue structure and function.
Fiorentino, who was invested as the inaugural holder of the Karl and Mary Fessenden Professorship in Biomedical Engineering this past fall, is the most recent faculty member in UVM's College of Engineering and Mathematical Sciences (CEMS) to receive a CAREER Award from the National Science Foundation (NSF). The prestigious award will help fund Fiorentino's ongoing research in the relationship between cartilage microstructure and the risk of a joint developing future musculoskeletal diseases such as osteoarthritis.
"Nic is an outstanding Mechanical Engineering faculty member in every category: teaching, research, and service; but his true impact is measured by the number and quality of the testimonies provided by his advisees and mentees for his tenure case," said Professor and Chair of the Department of Mechanical Engineering, Douglas Fletcher.
An improved understanding of tissue microstructure is only part of the equation. The proposed investigations hope to advance the field of cartilage biomechanics by quantifying microstructure and function across different age groups and varying degrees of joint degradation.
"While the relationship between microstructure and future cartilage function remains an open question, providing accurate measurements and benchmarks could profoundly impact our scientific understanding of cartilage tissue and benefit patients at high risk for developing osteoarthritis," said Fiorentino.
The Centers for Disease Control and Prevention (CDC) estimates that 1 in 5 (or 53.2 million) US adults have some form of arthritis. A potentially debilitating disease characterized by loss of articular cartilage and resulting joint stiffness and pain, osteoarthritis (OA) is the most common form of arthritis, affecting over 30 million adults in the United States or roughly 13% of the nation's adult population.
While chronic pain and disability are well-known symptoms of OA for the patient, the overall economic burden to the nation is equally daunting at an estimated $136 billion annually — a figure that has more than doubled in the last decade. OA is marked by the loss of cartilage, and once this loss begins, there are no treatments to reverse the damage. Many osteoarthritis patients endure the pain until its severity necessitates a joint replacement, with an estimated 1 million knee and hip replacements completed each year.

Fiorentino, who leads UVM's Musculoskeletal Imaging and Orthopaedic Biomechanics (MIOB) research laboratory, hopes his research can provide the required data and scientific understanding of cartilage tissue to, one day, help identify an individual's risk for developing osteoarthritis (OA). "A central focus of this research is better understanding cartilage microstructure. We know that cartilage changes before people get osteoarthritis, but we don't necessarily know, once it does change, how that alters the behavior of cartilage," said Fiorentino. That's really what we are trying to not only understand but also scientifically measure."
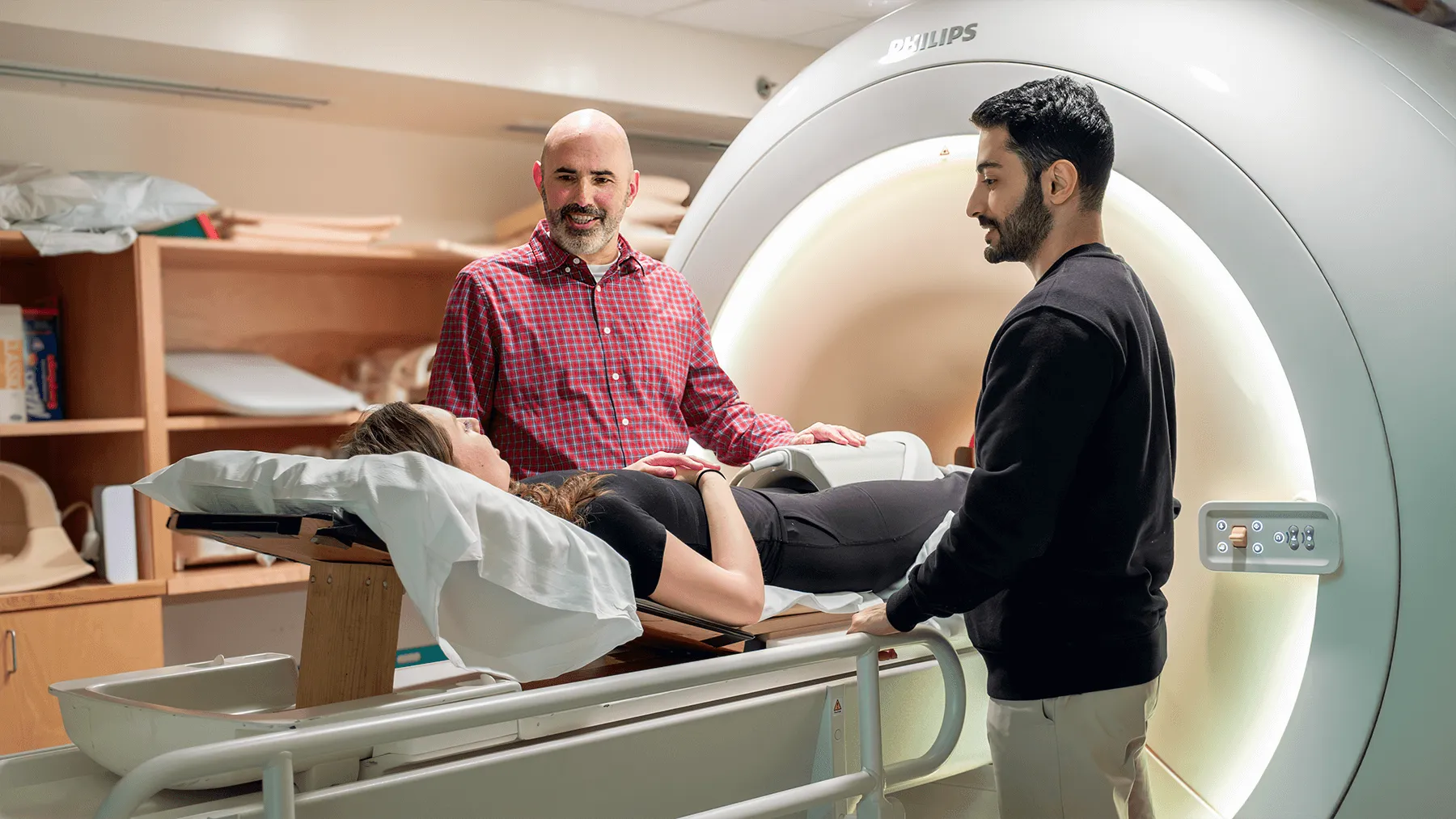
Recent advancements in quantitative magnetic resonance imaging (qMRI) offer a promising avenue for researchers like Fiorentino, in collaboration with colleagues at the Larner College of Medicine's MRI Center for Biomedical Imaging, to go beyond traditional qualitative MRI—which focuses on visual interpretation—by allowing numerical measurements of tissue properties taken at the pixel level of a scan.
By using the accurate measurements obtained with qMRI, Fiorentino hopes to establish a clearer understanding of how cartilage's microstructure relates to its function when both relaxed and under load. To achieve this, his proposal includes research objectives, including in vivo imaging and analysis on a broad range of living subjects and in situ experiments on cadavers, where the loads can be increased more rapidly in a controlled and precise manner.
The first objective focuses on participants with healthy knees, spanning ages 20 to 59, to capture a wide range of microstructural measurements. While most MRIs require absolute stillness on the part of the patient, the in vivo MRI scans incorporate a mechanical, pneumatic-driven loading device developed by Fiorentino and a cross-college design team. Cartilage health and function can be assessed by observing changes in qMRI values while applying different degrees of pressure to the participant's foot to measure the tissue under specific loads.
A second research objective aims to create and benchmark a computational modeling framework that uses finite element modeling to update material parameters based on qMRI measurements. This process will include in situ experiments, in which researchers can run identical MRI sequences with cadavers followed by mechanical tests with higher loads than those that can be prescribed during the in vivo scans.
Typically used in fields like structural mechanics and fluid dynamics, finite element modeling (FEM) is a numerical method used to develop solutions for complex engineering problems by breaking down large systems into smaller, discrete parts called finite elements. The models can be used to create a digital twin of a subject's knee and further simulate how the joint responds to different experimental scenarios, which can be later verified with in situ measurements.
Fiorentino's proposal also includes a series of educational and mentorship objectives that can be realized concurrently with the research objectives. One of the primary educational objectives is to organize a student-led engineering design team to further develop the knee joint loading device into a prototype that can be utilized in a clinical setting.
CEMS has a growing interdisciplinary design infrastructure, providing researchers with a robust capability to develop technological ideas into functioning prototypes. Among the many student-centered resources are senior capstone courses such as the Senior Experience in Engineering Design (SEED) program, where student teams design and engineer solutions to challenges provided by sponsoring companies, organizations, or—as in this case—UVM researchers and labs.
In addition, the college's Center for Biomedical Innovation is uniquely poised to further develop prototypes into marketable products with collaborative partnerships with the Larner College of Medicine, Grossman School of Business, and UVM Innovations, among others.
A wider educational objective of Fiorentino's project is to broaden and enhance students' perception of STEM disciplines and encourage them to explore STEM careers as they prepare for college by using established K-12 STEM-focused learning and outreach programs such as the 4-H Discover Engineering workshops and newly introduced CEMS-sponsored summer camps.
"This has been a huge thing for me. Bringing teens to campus to see our labs and discover the kinds of important research they can do," said Fiorentino. "The students get very interested when they have an opportunity to be immersed in the research process."
This exposure to science and engineering parallels Fiorentino's own journey to becoming a researcher and faculty member at the University of Vermont. As a young student-athlete, Fiorentino had an innate interest in physical performance and optimizing physical form. As an undergraduate student focusing on pre-med at the University of Florida, Fiorentino discovered how biomechanical engineering bridged his interests in medicine and performance.
"I was really drawn to biomechanics, the study of forces and mechanical engineering metrics and their application to the human body," said Fiorentino. "So, I decided I would prefer to help people with science."