Emeritus Professor — Inorganic and Analytical Chemistry
Research and/or Creative Works
1) Applications of chemically-modified electrodes
An important focus in our group is on development of methods for covalent attachment of redox-active molecules to electrode surfaces through an ethynyl group, thereby providing chemically-modified electrodes that can bridge the divide between homogeneous and heterogeneous electron-transfer processes. Transition metal complexes and organometallic compounds comprise the dominant classes of molecules to be attached, owing to their breadth of applications in redox catalysis and electro-analysis that take advantage of changes in the redox state of the metal center at different applied electrode potentials. Cheap and readily-available carbon is employed as the electrode material, thereby avoiding the sustainability problems inherent to the use of precious metal electrodes for electro-catalysis.
One of the methods we have developed for covalent attachment of ethynyl-linked compounds to carbon surfaces is based on the anodic oxidation of a compound containing a lithio-ethynyl group. As shown below for the case of a ferrocenyl derivative, 1, one-electron oxidation results in expulsion of a lithium ion and formation of a neutral ethynyl-based radical that forms a strong C-C bond with an atom on the electrode.
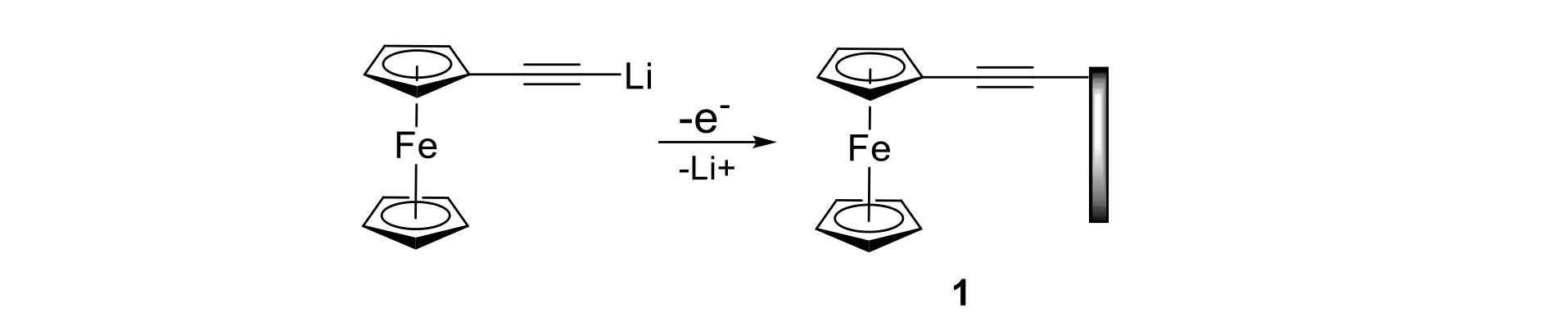
The redox activity of the resulting ferrocenyl-modified electrode is sustained for hundreds or more electron-transfer processes, showing that the molecule is attached permanently to the electrode surface. The cyclic voltammograms given here show that the currents for a monolayer of a ferrocenyl-modified electrode are virtually unchanged after 1000 scans (dark line).
Metal prophyrins comprise another class of compounds that we are attaching to carbon electrodes. Again, the molecule-to-surface linkage is an ethynyl group, a typical structure being 2. Development of metal-porphyrin based modified electrodes could provide new routes to applications in electron-transfer catalyzed reactions that are important to the development of fuel cells and other energy-transfer and -storage devices.
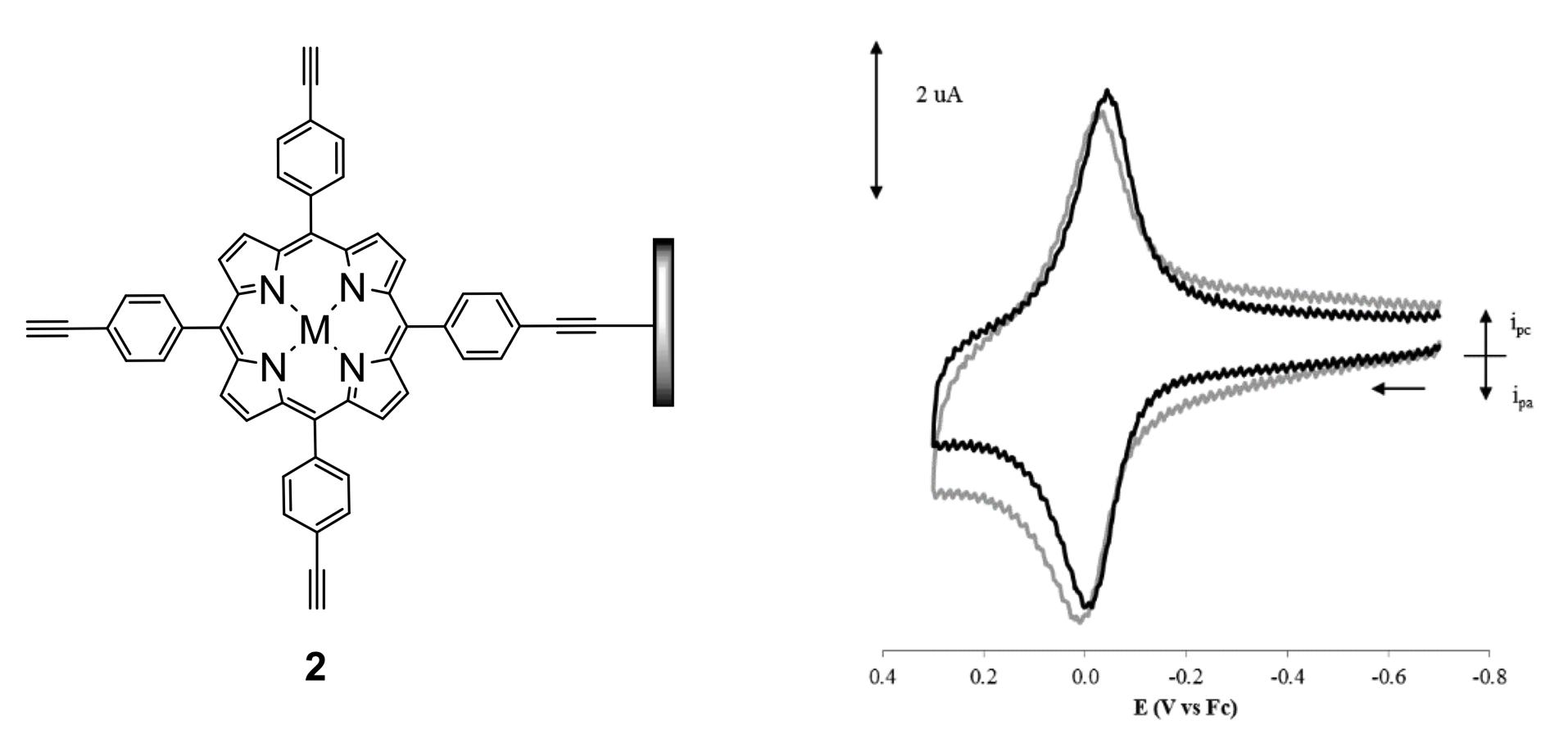
2) Organometallic Pharmaceuticals
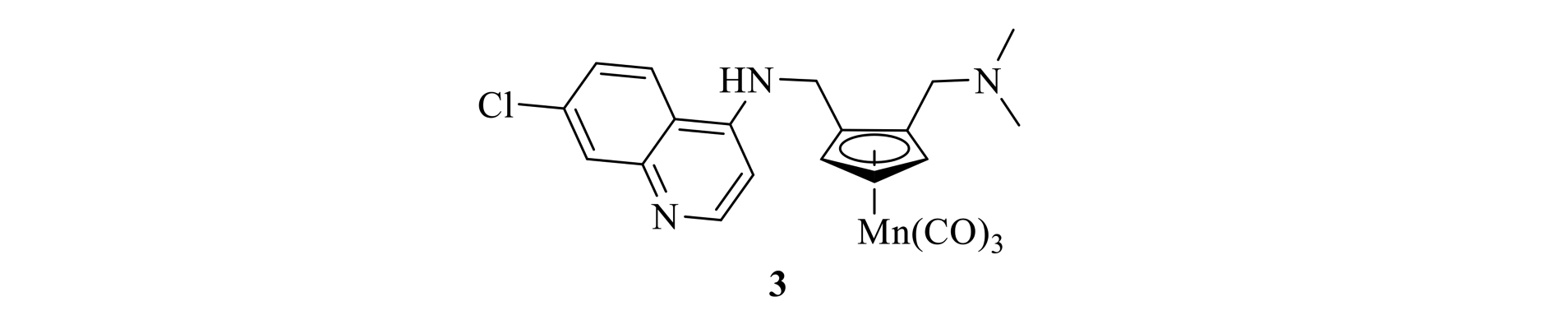
The development of a new family of pharmaceuticals is being investigated, in which a redox-active organometallic ‘tag’ is affixed to an organic fragment already known to have biological activity. The organometallic-tagged compounds may give access to new drugs of increased biological activity, reduced biological resistance, and increased analytical sensitivity. We are focusing on the so-called ‘cymantrene’ tag, in which a cyclopentadienyl manganese tricarbonyl moiety is linked to an organic substrate. For example, compound 3, prepared in our group, has shown significant activity against melanoma and other cancer cell lines
Publications
M. V. Sheridan, K. Lam, M. Sharafi, S. T. Schneebeli, W. E. Geiger. “Anodic Methods of Covalent Attachment of Ethynylferrocenes to Electrode Surfaces: Comparison of Ethynyl Activation Processes” Langmuir 2016, 32, 1645–1657.
K. Lam, W. E. Geiger. “Synthesis and Anodic Electrochemistry of Cymanquine and Related Complexes” J. Organometal. Chem. 2016, 817, 15–20.
M. V. Sheridan, K. Lam, W. E. Geiger. “An Anodic Method for Covalent Attachment of Molecules to Electrodes Through an Ethynyl Linkage” J. Am. Chem. Soc. 2013, 135, 2939–2942.
M. V. Sheridan, K. Lam, W. E. Geiger. “Covalent Attachment of Porphyrins and Ferrocenes to Electrode Surfaces Through Direct Anodic Oxidation of Terminal Ethynyl Groups” Angew. Chem. Int. Ed. 2013, 52, 12897–12900.
W. E. Geiger. “One-Electron Electrochemistry of Parent Piano-Stool Complexes” Coord. Chem. Rev. 2013, 257, 1459–1471.
F. Barriére, W. E. Geiger “Organometallic Electrochemistry Based on Electrolytes Containing Weakly-Coordinating Fluoroborate Anions” Accounts Chem. Res. 2010, 43, 1030–1039.
W. E. Geiger “Organometallic Electrochemistry: Origins, Development, and Future” Organometallics 2007, 26, 5738–5765.
D. R. Laws, D. Chong, K. Nash, A. L. Rheingold, W. E. Geiger “The Cymantrene Radical Cation Family: Spectral and Structural Characterization of the Half-Sandwich Analogues of Ferrocenium Ion” J. Am. Chem. Soc. 2008, 130, 9859–9870.
R. J. LeSuer, W. E. Geiger “Improved Electrochemistry in Low-polarity Media Using Tetrakis(pentafluorophenyl)borate Salts as Supporting Electrolytes” Angew. Chem. Int. Ed. 2000, 39, 248–250.
N. G. Connelly, W. E. Geiger “Chemical Redox Agents for Organometallic Chemistry” Chem. Rev. 1996, 96, 877–910.
Awards and Recognition
Pomeroy Professor, University of Vermont; University of Vermont Scholar; Dean’s Lecturer, University of Vermont; Erskine Fellow, University of Canterbury; Leverhulme Fellow, University of Bristol; Graduiertenkolleg Fellow, University of Freiburg; Outstanding Alumnus, Canisius College; Member, Vermont Academy of Science & Engineering.

Areas of Expertise and/or Research
Molecular electrochemistry; organometallic electrochemistry; electron-transfer induced changes in molecular structure and reactivity; molecular modification of electrode surfaces; organometallic pharmaceuticals.
Education
- B.S., Canisius College, Buffalo, NY, 1965
- Ph.D., Cornell University, Ithaca, NY, 1969
- Research Associate, University of California at Riverside, Riverside, CA, 1968–1969
- Postdoctoral Research Associate, Northwestern University, Evanston, IL, 1969–1970
Contact
- (802) 656-0268
Innovation E346
