Center Members Early Career Awards
Diego Adrianzen-Herrera, MD, and Mansour Gergi, MD, were recipients of the Early Career Green and Gold Professor of Medicine award, and Osama Harraz, PhD, was awarded the Bloomfield Early Career Professor award in Cardiovascular Research.
Learn more about Dr. Diego Adrianzen-Herrera's research Learn more about Dr. Mansour Gergi's research Learn more about Dr. Osama Harraz's researchVermont Center for Cardiovascular and Brain Health Development
Grants Awarded to Center Members from External Sources

External Grants Awarded To Center Members
• Research Mentorship Award granted by the HTRS, totaling $165,000 in 2021.
• R01 research grant awarded by the NIH/National Heart, Lung, and Blood Institute (NHLBI), totaling $2,754,805 allocated in 2023.
• Research Scholar Program granted by the NNE-CTR.
• Bloomfield Professorship Award granted by the CVRI, totaling $135,000 in 2023.
• NIGMS COBRE (Centers of Biomedical Research Excellence) Supplement—Team Science Supplement awarded by the CVRI, totaling $660,479 in 2023.
• Pilot Grant funded by the Chan-Zuckerberg Foundation Collaborative Pairs Program, totaling $200,000 in 2023.
• R01 Research Project Grant awarded by the NIH/NHLBI, totaling $2,898,240 in 2022.
• LeWinter Young Investigator Award granted by the CVRI, totaling $5,000 in 2022.
• R21 Exploratory/Developmental Research Grant awarded by the NIH/NIA, totaling $358,224 in 2022.
Saul Huerta de la Cruz, PhD
• Postdoctoral Fellowship awarded by the American Heart Association for a total of $144,580 in 2023.
Abbie Johnson, PhD
• R01 research grant awarded by the NIH for a total funding amount of $1,642,000 in the year 2021.
• AHA Postdoctoral Fellowship granted by the American Heart Association, amounting to a total of $140,952 in the year 2022.
• Career Development Award awarded by the American Heart Association, totaling $231,000 in funding for the year 2022.
• R01 Grant awarded by the NIH, awarded totaling $2,773,407 in 2025.
• 2020 Million Dollar Bike Ride grant awarded by the UPenn Orphan Disease Center, totaling $82,795 in 2020.
Maria Noterman Soulinthavong, PhD
• Diversity Administrative Support funding was awarded by the NIH, totaling $255,411, in the fiscal year 2023.
• L30 grant funded by NIH/NINDS, with a total awarded amount of $27,724.
• L30 grant funded by the NIH/NINDS, with a total awarded amount of $17,114.
• NSF Career Grant awarded by the NSF for a total of $665,769 in 2025.
• R35 MIRA grant awarded by NIGMS, totaling $1,250,000 in fiscal year 2022.
Emmett Whitaker, MD
• R35 Grant awarded by the NIGMS totaling $1,950,000 in 2022.
• COBRE Supplement awarded by the NIH/NIGMS totaling $272,427 in 2022.
Grants Awarded to Center Members from Internal Sources

Internal Grants Awarded To Center Members
• Pilot Grant awarded by the University of Vermont Department of Medicine, for a total of $50,000 in 2022.
• Pilot grant awarded by the University of Vermont Department of Medicine, totaling $50,000, awarded in 2023.
• Pilot grant awarded by the University of Vermont Early Career Professorship, amounting to $10,000, awarded in 2023.
• University of Vermont Cancer Center Pilot Grant awarded by the University of Vermont Cancer Center, for a total of $20,000 in 2023.
Timothy Plante, MD
• Bloomfield Early Career Professorship granted by the University of Vermont, for a total of $120,000 in 2021.
Maria Noterman Soulinthavong, PhD
• Early Career Research Award granted by the University of Vermont CVRI, for a total of $7,371 in 2023.
Center Growth

Since the establishment of our center in 2020, we have cultivated a multidisciplinary community of more than 60 investigators from a broad array of research backgrounds. We have successfully recruited six assistant professors and three associate professors across four distinct departments and have funded nine Research Project Leaders and nine Pilot Project Leaders. Our Science Cores have expanded their services to the greater UVM community and beyond to investigators from other institutions outside of Vermont.
View the projects of our Active Research Project LeadersView the projects of our Past Research Project Leaders
Research Spotlights Featuring VCCBH Members

R1 Larner Spotlight: New Evidence from Larner Researchers Points to Potential Treatment for Vascular Dementia

R1 Larner Spotlight: New Heart Health Research Under Way at UVM
R1 Larner Spotlight: Grant Supports Punihaole’s Novel Cell Imaging Technique

R1 Larner Spotlight: Koide Lab Explores Links Between Blood Pressure Regulating Hormone and Cognitive Decline

R1 SPOTLIGHT: Mark Nelson - Understanding the Brain's Regulation of its Blood Flow
Supporting 100+ UVM researchers in experimental design, biostatistics, and imaging for 90 projects, yielding 41 peer-reviewed articles.
Explore Our Cores
Accolades & Accomplishments featuring Center Members
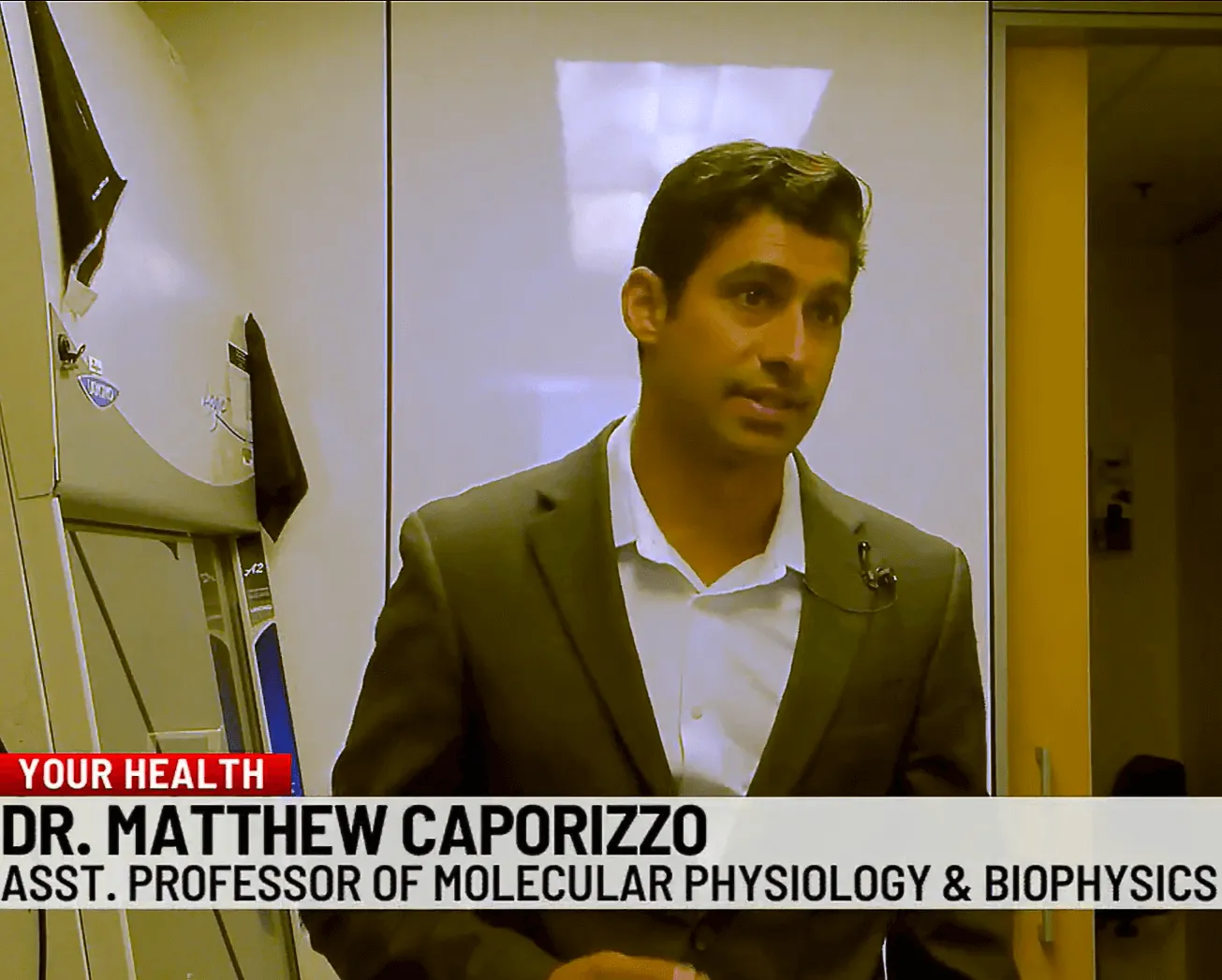
Matthew Caporizzo, Ph.D.

Heart disease, including heart failure, has been the leading cause of death in the U.S. since 1950. About 50 percent of the 5 million Americans with heart failure have heart failure with preserved ejection fraction (HFpEF), where the heart’s left ventricle becomes stiff and doesn’t relax properly. Despite a normal ejection fraction, the blood volume pumped is inadequate due to poor ventricular filling.
Matthew Caporizzo, Ph.D., assistant professor of molecular physiology and biophysics, along with researchers at the University of Pennsylvania—in partnership with pharmaceutical company MT-Act—have developed a small molecule inhibitor to repair the heart’s microtubule network (MTN), addressing a key issue in HFpEF. Their recent paper, “Vasohibin Inhibition Improves Myocardial Relaxation In Heart Failure with Preserved Ejection Fraction,” describes the creation of a vasohibin inhibitor (VASHi) that blocks MTN detyrosination, showing promising results in live animals.
After successful trials in rats, Caporizzo’s team tested VASHi on isolated human heart cells from non-failing and failing hearts and found VASHi also improved relaxation in failing human heart cells. Excitingly, this suggests that VASHi could be a potential treatment to reduce stiffness and improve heart relaxation in HFpEF.
Katharine Cheung, M.D., Ph.D., M.Sc.
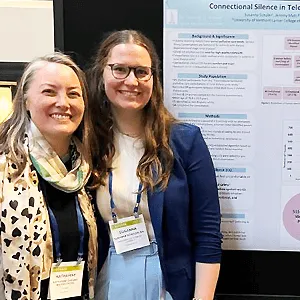
Katharine Cheung, M.D., Ph.D., M.Sc., interim director of the UVM Center on Aging, associate director for research, and assistant professor of medicine, and her mentee, medical student Susanna Schuler ’26, presented their research findings at the American Academy of Hospice and Palliative Medicine State of the Science meeting on March 23 in Phoenix, Arizona.
Cheung conducted a two-year pilot clinical trial of tele-palliative care for rural dialysis patients funded by the National Palliative Care Research Center. Schuler received a Larner College of Medicine Summer Research Fellowship to study connectional silence using human and machine learning in the recordings of palliative care conversations with dialysis patients. Her results demonstrate that connectional silence, which is associated with quality communication, can be found in telehealth similar to in-person visits, but there are differences in the types of silence detected by humans compared to the machine learning algorithm. Her work was supported by the UVM Center on Aging.
Learn more about Katharine Cheung, M.D., Ph.D., M.SRead the full Center on Aging newsletter
Mary Cushman, M.D., M.Sc.
Mary Cushman, M.D., M.Sc., University Distinguished Professor of medicine and pathology & laboratory medicine, was invited to present at the International Society on Thrombosis and Haemostasis (ISTH) congress in June in Bangkok, Thailand. Her project, “Circulating C-reactive Protein and D-dimer Concentrations and Risk of Severe Covid-19: the Collaborative Cohort of Cohorts for Covid-19 Research (C4R),” details preliminary findings from the C4R study.
The C4R project combined 14 longitudinal studies to explore various questions about COVID-19 risk factors and outcomes. In five of these studies—the Framingham Heart Study, the Atherosclerosis Risks in Communities Study, the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study, the Jackson Heart Study, and the Multi-Ethnic Study of Atherosclerosis—Cushman led a team that used laboratory data from 20 years prior to the pandemic to examine the association between thrombo-inflammation status and the risk of severe COVID illness.
Participants were, on average, 75 years old when the pandemic started. Cushman’s team found that people with higher pre-pandemic C-reactive protein—reflecting higher inflammation status—developed more severe illness if they became infected with COVID. People with higher D-dimer, a prothrombotic risk factor, did not show this relationship. Further study is needed to identify the reasons why inflammation status 20 years before the pandemic relate to greater susceptibility to severe COVID.
The research for this project was funded through the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging.
Mary Cushman, M.D., M.Sc., Neil Zakai, M.D., Nels Olson, Ph.D., and Debora Kamin Mukaz, Ph.D.
Investigators at the Larner College of Medicine will continue their 23-year program studying stroke and cognitive disorders in the United States, thanks to a $10.1 million multi-year grant from the National Institutes of Health (NIH) renewing a REGARDS (REasons for Geographic and Racial Differences in Stroke) study grant. The purpose of the REGARDS project is to understand why people in some parts of the country develop more strokes than people in other parts of the country, and why blacks develop more strokes than whites.
The study followed 30,239 Black and White adults over the age of 45 from the lower 48 states. The findings have been significant, resulting in over 750 academic papers based on REGARDS data. One striking discovery revealed that Black people under age 75 are more than twice as likely to die from stroke than white people. Another key finding showed that people living in the South had a 40 percent higher risk of stroke-related death compared to those in other parts of the U.S. It also noted that Alzheimer’s Disease and Related Dementia (ADRD) occur more frequently in Black Americans. While REGARDS investigators have uncovered a litany of reasons for these differences, unfortunately, trends in adverse health in Black Americans seem to be worsening over time.
Due to the remarkable success of this study, the NIH has renewed its grant, allocating $16.4 million in the next two years, and $37.1 million over the next five years, to support this multi-institutional project. Of this, UVM will receive $4.3 million in the initial two years, and over $10 million by the end of the fifth year. This next step will focus on investigating factors contributing to worsening health disparities among Black Americans, the rise in stroke mortality since 2014, and effects of these trends on dementia and brain health.
“It is alarming that our population’s health is worsening, and that Black Americans continue to have poorer health outcomes than others,” said Mary Cushman, M.D., M.Sc., University Distinguished Professor at UVM and co-leader of the REGARDS collaboration. “This new research will help provide answers to narrow the gap. Here at the UVM Larner College of Medicine, REGARDS has been an engine for our faculty and students conducting research in these areas for over 20 years, and I am excited that we will be able to continue this, and improve the health of our country.”
REGARDS plans to recruit a new group of 12,000 Black and white adults between the ages of 45 and 64, and will continue to follow 8,000 original participants of the study. The study will compare people born in 1908–1960 and enrolled in middle age in 2003–2007 with people born in 1961–1980 and enrolled in middle age now. This will allow researchers to understand how the uptick in obesity and risk factors have impacted brain health, specifically related to stroke and dementia. The team will also address increasing health disparities affecting Black Americans.
The study’s infrastructure has supported the research careers of Larner faculty like Neil Zakai, M.D., Tim Plante, M.D., and others. More than 35 trainees, including post-doctoral fellows, graduate students, residents, and medical students, have also benefited from the mentorship and research opportunities provided through UVM’s involvement with the REGARDS study.
Cushman also highlighted that REGARDS currently supports 133 active ancillary studies, funded outside of the primary study grant. These include grants awarded to Larner faculty members, such as Nels Olson, Ph.D., and Debora Kamin Mukaz, Ph.D. To date, UVM faculty working in REGARDS have secured over $33 million in funding for the university—not including the current award.
The REGARDS study would not be possible without the work of key staff members at the Larner College of Medicine, including Elaine Cornell, Rebekah Boyle, Jill Sanders, and Nicole Gagne.
Learn more about Mary Cushman, M.D., M.SLearn more about Neil Zakai, M.D.
Osama Harraz, Ph.D.

While it’s well established that increased blood flow (hyperemia) is vital for brain function—delivering oxygen and nutrients, clearing waste, and supporting neural activity—what happens after this surge is less understood. In a recent paper published in Nature Communications titled “Endothelial Piezo1 Channel Mediates Mechano-Feedback Control of Brain Blood Flow,” Osama Harraz, Ph.D., Bloomfield Early Career Professor in Cardiovascular Research and assistant professor of pharmacology at the Robert Larner, M.D. College of Medicine, and his team of researchers from American and European institutions reveal that Piezo1, a lesser-understood protein, acts as a “brake” system, helping blood flow return to normal after neural activity.
Functional hyperemia (FH) refers to the process by which blood flow to a specific area, such as the brain, increases in response to activity. For instance, when a region of the brain becomes more active—like when you’re thinking or moving—it requires more oxygen and nutrients. To meet this demand, blood vessels widen, allowing more blood to flow to that area, which helps the active tissues function optimally. Piezo1, a sensor located in the cells lining these blood vessels, detects physical forces, such as friction, caused by the increased blood flow.
To investigate Piezo1’s role in controlling blood flow and its impact on cognition, Harraz and his colleagues used genetically engineered mice to manipulate Piezo1’s function. They discovered that Piezo1 acts like a “brake,” facilitating the return of blood flow to normal levels after a spike. When Piezo1’s function was altered, the mice struggled with memory tasks, indicating that Piezo1 is crucial not only for regulating blood flow but also for cognitive function.
To test his hypothesis, Harraz applied a Piezo1 activator—called Yoda1—to the subjects’ brains while stimulating their whiskers. They noted a significant decrease in blood flow, measured by laser Doppler, after Yoda1 was applied, with no changes in baseline blood flow or blood pressure. To further confirm their findings, the team employed imaging techniques to monitor blood vessels during the whisker stimulation and discovered a significant reduction in blood vessel dilation.
Their results confirmed that Piezo1 plays a key role in regulating the decline in blood flow after stimulation: mice with heightened Piezo1 activity showed faster recovery of blood flow, while those lacking Piezo1 experienced slower recovery. Additionally, enhanced Piezo1 activity slowed the initial rise in blood flow, whereas the absence of Piezo1 accelerated it. Harraz concluded that activating Piezo1 in endothelial cells—the cells lining the blood vessels—reduces the brain’s blood flow response without altering the structure of the blood vessels or other brain cells.
“There is a growing number of human Piezo1 mutations. The mutant mice used in our study carry a human Piezo1 mutation that causes hemolytic anemia, and other Piezo1 mutations are prevalent in people of African descent, including African Americans,” said Harraz. “Brain blood flow is essential for cognition and memory, and our discovery reveals that Piezo1 determines blood flow dynamics. Therefore, it will be important to explore whether human Piezo1mutations adversely impact brain function and could be risk factors for dementia.”
Harraz’s research highlights the critical role of Piezo1 in neurovascular coupling—the communication between neurons and blood vessels—and underscores the importance of its function for both blood flow regulation and cognitive health.
Debora Kamin Mukaz, Ph.D.

On November 16, Larner Assistant Professor of Medicine Debora Kamin Mukaz, Ph.D., M.S., moderated a panel on science policy advocacy titled “How can we engage scientists from historically underrepresented backgrounds in policymaking and advocacy?” at the American Heart Association’s Scientific Sessions 2024 conference in Chicago. The panelists were AHA President Keith Churchwell, M.D.; past AHA President Michelle Albert, M.D., M.P.H.; Emelia Benjamin, M.D., Sc.M., associate provost and professor of medicine and epidemiology at Boston University; and Carl Streed, Jr., M.D., M.P.H., associate professor of medicine at Boston University.
The focus of the panel was on how scientific research and medicine inform laws and policies at the local, state, and federal levels but scientific and medical engagement in policymaking and advocacy remains limited. The panelists discussed how scientists and clinicians—particularly early-career scientists and clinicians from historically underrepresented communities—can increase the policy impact of their work, build long-term relationships with policymakers (i.e., lawmakers), and communicate scientific and clinical evidence to policymakers in a clear and concise manner.
Mark Nelson, Ph.D.

Mark Nelson, Ph.D., chair and University Distinguished Professor of pharmacology, has been selected to serve a two-year term on the Advisory Committee to the Director of the National Institutes of Health (NIH).
The advisory committee advises on matters related to planning, execution, conduct, support, and evaluation of biomedical research, medical science, and biomedical communications; makes recommendations concerning program development, resource allocation, NIH administrative regulation and policy, and other specific or general aspects of NIH policy; and reviews and makes recommendations on applications for grants and cooperative agreements for research and training for projects that show promise of making valuable contributions to human knowledge.
Denise Peters, Ph.D., D.P.T., P.T. and David Jangraw, Ph.D., M.S.

A collaborative research team co-led by investigators David Jangraw, Ph.D., M.S., and Denise Peters, Ph.D., D.P.T., PT, has been awarded the 2024 Armin Grams Memorial Research Award by the Center on Aging. The project will assess in-home instruments designed to measure mobility, activities of daily living, and speech production in aging rural Vermonters with and without mild cognitive impairment.