
Project Director: Diego A Adrianzen, M.D.
Assistant Professor of Medicine
Department of Medicine-Hematology Oncology
University of Vermont
Project: Risk Prediction and Inflammatory Biomarkers of Cardiovascular Disease in Myelodysplastic Syndromes
Project Description
Myelodysplastic Syndromes (MDS) are blood cancers caused by acquired mutations in cells of the bone marrow that give rise to blood cells (bone marrow progenitors). These mutations also cause excess inflammation in blood vessels and risk of cardiovascular diseases (CVDs), which are a large source of complications and mortality in patients with MDS. Dr. Adrianzen Herrera’s previous work has described that MDS patients have a high risk for CVD and death from CVD. His current project has two goals. First, he will develop and validate a clinical tool to predict risk of CVD in patients with MDS. Second, he will study biomarker changes in the blood in people with MDS. The ultimate goal is to understand why people with MDS develop CVD and how this can be prevented.
Project Figure
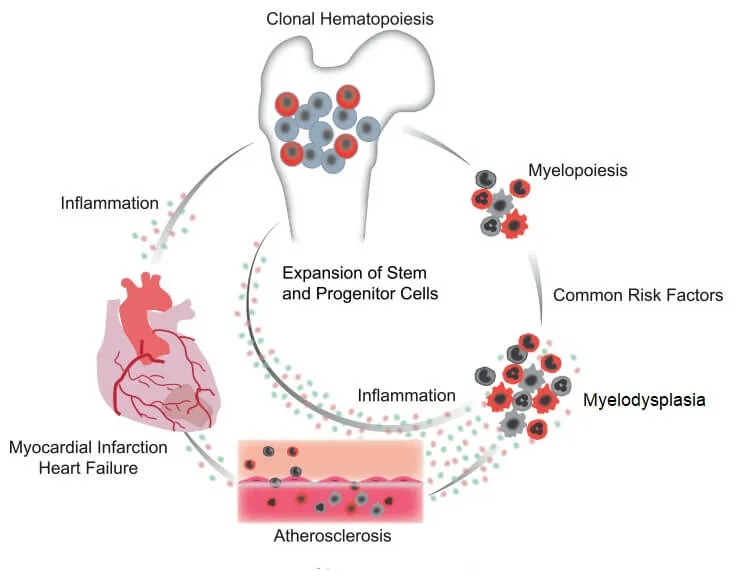
PDF describing image: Role of myelodysplastic syndromes in cardiovascular disease
Project Mentorship Team
Senior Mentor
Neil Zakai, MD, MSc
Senior Mentor
Ira Bernstein, MD
Peer Mentor
Nels Olson, PhD
External Mentor
Pamela Lutsey, PhD, MPH