Project Director: Matthew Caporizzo PhD
Assistant Professor
Department of Molecular Physiology and Biophysics
Extracellular Drivers of Myocyte Stiffening in Diastolic Heart Disease
Project Description
Diastolic heart disease arises from combined hypertension and metabolic syndrome, and it is the leading cause of death and disability worldwide. Diastole depends on a heart’s stiffness and ability to relax or to reduce active tension. Diastolic dysfunction is the hallmark of diastolic heart disease. However, its molecular underpinnings are controversial and are thought to involve sarcomeres (the contractile unit of muscle), the microtubule network (a cellular mesh of stiff cytoskeletal filaments) and extracellular fibrosis. The role of the microtubule network in diastolic heart disease remains largely unexplored; however, data from heart failure studies show that the microtubule network regulates the stiffness and relaxation of cardiomyocytes. We hypothesize that the microtubule network contributes to cardiomyocyte-level stiffening and diastolic dysfunction in diastolic heart failure. To test this, we will use rat models of hypertension and diastolic heart disease to evaluate microtubule network-based stiffening. We will combine our unique expertise in microtubule network structural dynamics, proteomics and mechanics with a novel working myocardial preparation to evaluate the contribution of sarcomeres, fibrosis and the microtubule network on myocardial diastolic performance (Fig 1). We anticipate that only rats with diastolic heart failure will exhibit impaired relaxation. Our ultimate goal is to determine the molecular basis of diastolic dysfunction and the potential of microtubule network-based therapies for hypertension and diastolic heart disease.
Project Figure
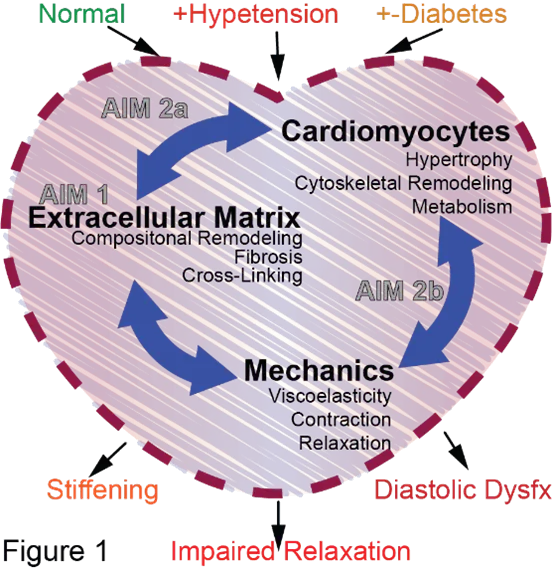
PDF describing image: Role of the extracellular matrix in normal and diseased heart

Project Director: Katharine Cheung, MD, PhD
Assistant Professor
Department of Medicine- Nephrology
Tenure-track
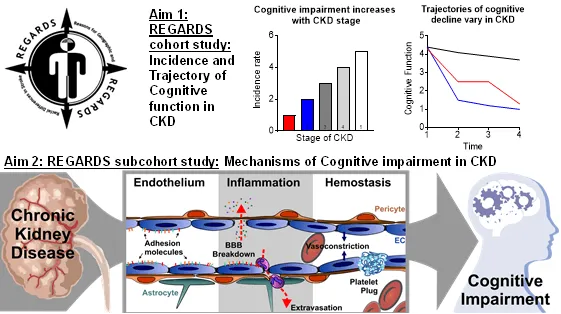
Vascular Mechanisms of Cognitive Impairment in Chronic Kidney Disease
Project Description
Chronic kidney disease (CKD) affects 30 million people in the U.S. and is independently associated with a 2-fold increased risk of cognitive impairment, especially vascular-mediated cognitive impairment. CKD causes disturbances in the vascular compartment including higher levels of inflammation, hemostatic and endothelial activation biomarkers. We hypothesize that the pathophysiological processes that these biomarkers represent are implicated in the development of vascular cognitive impairment via reduction in cerebral blood flow, and altered permeability of the blood brain barrier, leading to tissue damage. The proposed research will study participants in the REGARDS Study, a nationally representative biracial cohort of 30,239 individuals followed for over 10 years with repeated cognitive assessments. Dr. Cheung will harness these rich longitudinal data to determine the trajectories of cognitive decline and to study the mechanisms of cognitive impairment in those with CKD. She will study biomarkers of inflammation, hemostasis, and endothelial activation as mediators of cognitive impairment in CKD.
Project Figure

Project Director: Osama Harraz, PhD
Assistant Professor
Department of Pharmacology
University of Vermont
Brain Capillary Mechanosensation by Piezo1 Channels in Health and Disease
Project Description
As blood moves through a narrow brain blood vessel, it imposes mechanical forces on the vessel wall, raising an important question: do capillaries (the very smallest blood vessels) sense the forces associated with flowing blood? This research will examine how blood vessels sense these forces and influence changes in blood flow in the mouse brain using genetically engineered mice and advanced imaging techniques. The ultimate goal of these studies is to provide results that lead to strategies for preventing deficits in brain blood flow in human diseases such as hypertension, which plays a major role in brain aging.
Project Figure 1
Project Figure 2
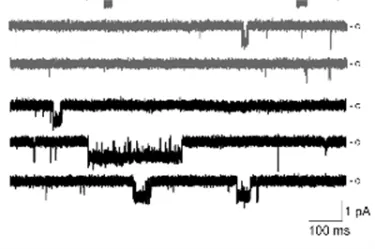
PDF describing image: Channel Activity in Brain Capillaries

Project Director: Debora Kamin Mukaz, PhD
Assistant Professor of Medicine
Department of Medicine- Hematology Oncology
University of Vermont
Project: Cardiovascular Health and Inflammation: Intersectional Impact of Structural Racism, Structural Sexism, and Structural Classism
Project Description
Poor cardiovascular health (CVH) is an important cause of heart disease and stroke. Inflammation from poor CVH may be a reason for this. In the U.S., people of different races, genders, income, and education levels have different CVH and levels of blood measures of inflammation. This may be due to structural oppressions like racism, sexism, and classism. Dr. Kamin Mukaz's research will reveal how structural racism, sexism, and classism are linked to CVH and inflammation in Black and White women and men of the U.S. REGARDS study. She will look over 10 years at the effects of structural oppressions on CVH and on 92 blood measures of inflammation.
Project Figure
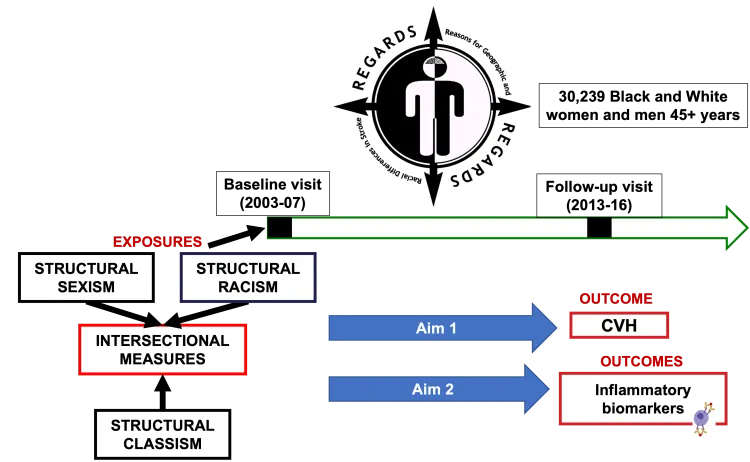
PDF describing image: Role of structural oppressions on inflammation and cardiovascular health

Project Director: Nicholas Klug, PhD
Assistant Professor
Department of Pharmacology
Role of brain capillary KATP Channels in Physiological Deep Brain Hypoxia Sensing and Deficient Blood Flow Regulation in Ischemic Small Vessel Disease
Project Description
The main objectives of this proposal are to determine if blood flow responses mediated by capillaries in deep brain structures are altered during chronic hypoxia and whether deep brain hypoxia-sensing is disrupted in an animal model of cerebral small vessel disease. These questions will be addressed using sophisticated deep brain ultrasound imaging in well-characterized mouse models of cerebral small vessel disease.
Project Figure 1
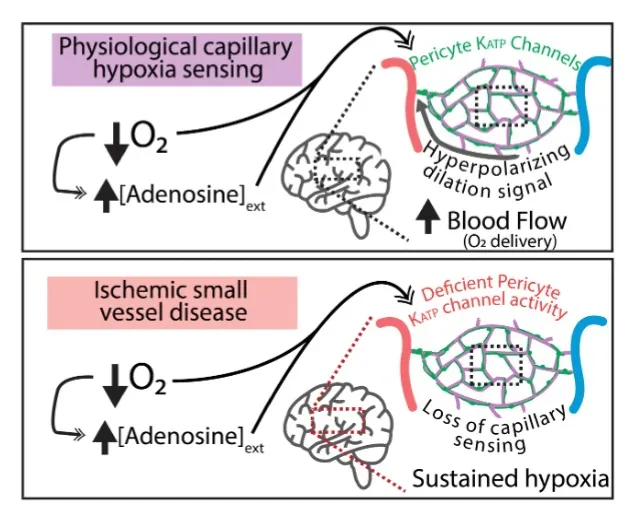
PDF describing image: Oxygen sensing by brain capillaries in health and disease

Project Director: Masayo Koide, PhD
Research Assistant Professor
Department of Pharmacology
Crippled Cerebral Blood Flow Regulation in Chronic Hypertension
Project Description
Cerebral blood flow is exquisitely controlled to meet the diverse and ever-changing demands of active neurons throughout the brain. This moment-to-moment adjustment of local blood flow, observed as increased cerebral blood flow within active regions of brain, is known as functional hyperemia. Dr. Koide’s recent work has demonstrated a novel signaling pathway, capillary-to-arteriole electrical signaling, which is a major contributor to functional hyperemia. Her proposed research will examine 1) the impact of chronic hypertension on functional hyperemia, specifically on capillary-to-arteriole electrical signaling, and 2) the molecular mechanism of impaired functional hyperemia, centering on plasma aldosterone as a crucial player, in a murine model of polygenic hypertension. Hypertension is the leading risk factor for cardiovascular and cerebrovascular diseases and affects an estimated 80 million people in the United States and 1 billion individuals worldwide. Although a growing body of evidence indicates that hypertension attenuates functional hyperemia, and contributes to impaired cognitive function in humans as well as in hypertensive animals, the mechanisms underlying this pathophysiological process have yet to be elucidated.
Project Figure
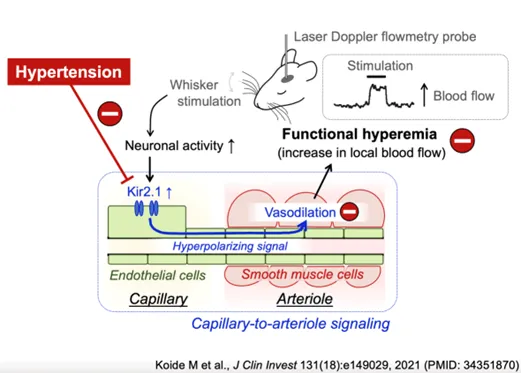
PDF describing image: Neural stimulation of cerebral blood flow in health and hypertension

Project Director: Denise Peters, PT, D.P.T., PhD
Associate Professor and DPT Program Director
Department of Rehabilitation and Movement Science
Doctor of Physical Therapy Program
Investigation of Clinical, Blood, and Neuroimaging Biomarkers as Predictors of Independent Walking Post-stroke
Project Description
More than half of stroke survivors experience difficulty with walking, contributing to disability, loss of independence, and increased caregiver burden. The long-term repercussions of loss of independent walking, along with limited rehabilitation time and resources, demonstrates an urgent need for advancing prediction of early walking recovery to improve patient-centered care and support discharge planning. The proposed research seeks to elucidate prognostic biomarkers in persons who have had a stroke for predicting walking recovery at 3 months after stroke. Measures will be obtained within the first week after stroke including clinical tests of strength and balance, MRI scans to assess the structural integrity and connectivity of brain pathways important for movement, and blood draws to examine blood markers related to recovery and repair. Early identification of patients with the potential to recover independent walking can allow for tailored interventions to assist recovery and positively effect quality of life.
Project Figure 1

Project Figure 2
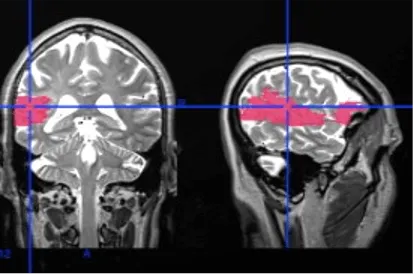
PDF describing image: Magnetic resonance image of a patient’s brain after stroke.

Project Director: David Punihaole, Ph.D.
Assistant Professor
Department of Analytical Chemistry, Physical Chemistry, Biophysical Chemistry
University of Vermont
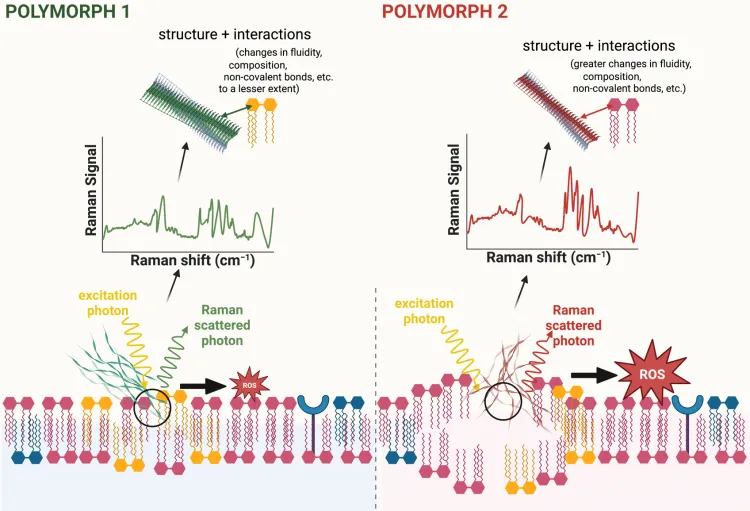
Determining How Amyloid-Beta Fibril Polymorphism Influences Cellular Toxicity
Project Description
Cerebral Amyloid Angiopathy (CAA) and Alzheimer’s Disease (AD) are debilitating diseases that can lead to memory loss, cognitive decline and dementia. A pathological hallmark of CAA and AD is the formation of plaques composed of amyloid-β (Aβ) fibrils in blood vessels and brain. Structural variants of fibrils, termed polymorphs, have been detected in human tissues and are thought to contribute differently to the pathophysiology of these diseases. However, the causal relationship between fibril structure and cellular toxicity remains largely unknown. To evaluate this relationship, we will use novel chemical imaging and electrochemical sensing methods to directly monitor the structural dynamics, aberrant interactions, and toxic activities of different Aβ fibril polymorphs in human tissues and animal models of CAA. Successful completion of this project will ultimately provide information that guides the rational design of drugs to limit the formation and toxic interactions of Aβ fibril polymorphs in people with CAA and AD.