Whether you opt for a general over-the-counter kit or the more precise UVM laboratory test available at http://go.uvm.edu/soiltest, one soil test result to pay attention to is pH.
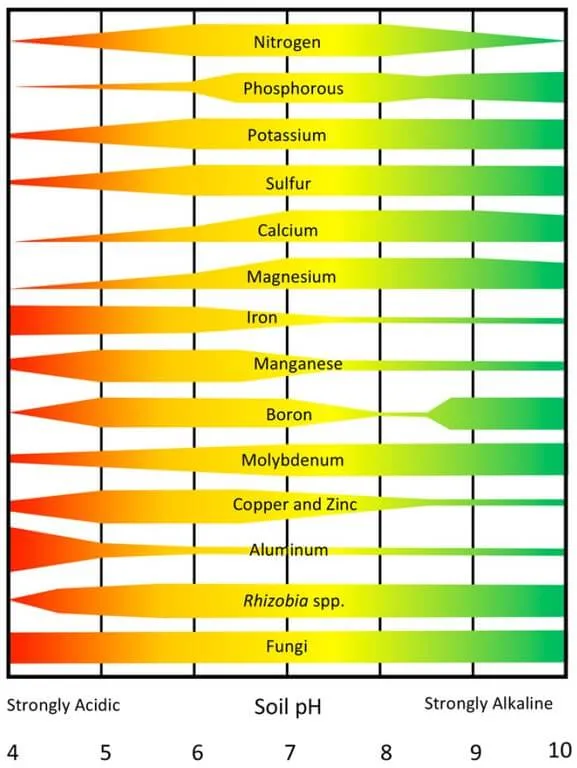
Soil pH is the measure of how acidic or alkaline your garden soils are. It is reported on a scale of 0 to 14 where 0 is low or the most acidic (the equivalent to battery acid) and 14 is high or the most alkaline (equivalent to lye). Soil pH 7.0 is considered neutral.
Knowing your soil’s pH is critical because it can have a profound effect on the availability of nutrients already in your garden. Many plants will adequately grow in soils with a pH of 6.0 to 7.5. However, the ideal pH for most vegetable and berry crops is between 6.5 and 6.8. The exceptions are plants like blueberries and rhododendrons that prefer more acidic soils with a soil pH around 4.5 to 5.5.
While soil test results can also reveal the levels of nutrients like phosphorus and potassium in your soils, it is soil pH that determines how much of those nutrients are available to your plants.
Let’s take phosphorus (P) as an example. Soil pH significantly impacts P availability. The ideal pH for optimal plant uptake of P is between 6.0 to 7.0. If soils are too acidic, phosphorus reacts with other soil nutrients (in this case, aluminum and iron), essentially locking up the P your plants could potentially use. On the other hand, a soil with too high of a pH may lead to P reacting with calcium which reduces phosphorus availability to your plants. These chemical interactions occur with other soil nutrients too, each having their own reactions with other nutrients, resulting in optimal rates of availability for each nutrient.
The sweet spot, then, becomes a soil pH range where the majority of nutrients are optimally available to meet the nutritional needs of your garden plants. Our challenge as gardeners is to adjust soil pH to land in that optimal range of 6.5 to 6.8.
Since most Vermont soils are naturally acidic or become more acidic over time, we need to raise pH in many gardens. This is accomplished with the addition of lime. UVM Extension vegetable and berry specialist Vern Grubinger recommends using dolomitic lime when magnesium (Mg) levels are low, or high-calcium lime when Mg levels are high. It is important to only apply the amount of lime recommended in your soil test report. Too much lime can raise the soil pH excessively, leading to soils that are too alkaline. Often, recommendations include a split application. This means applying smaller amounts a couple of times, typically once in the spring and once in the fall. Wood ash can be used as an alternative to lime. Note that ash from treated wood should never be used in the garden.
If your soil test results suggest that soil pH is too high or alkaline, the addition of elemental sulfur is recommended. Be sure to follow the recommendations to avoid over-application.
Contact the UVM Extension Master Gardener Helpline at https://go.uvm.edu/gardenhelpline if you need assistance with your soil test results. If you carefully follow the recommendations, your soil pH will land in the sweet spot your plants will love.



