Step 1: How to access CITI
I would like to use my UVM Net ID
All UVM students, faculty and staff are issued a UVM Net ID, but there are many UVMMC (non-UVM) research key personnel who do not have a UVM Net ID. A UVM Net ID can be issued to UVMMC personnel to access the CITI Program Training. Processing time can take 1-2 business days.
Once you have an active UVM Net ID, then you may open the browser and go to the CITI Login Page.
Click “Log In” which is located at the top right of the CITI page.

Then, click "Log In Through My Institution" to view the organizations listed to use "Single Sign On" (SSO) for CITI Program access, scroll down to click on “University of Vermont”...
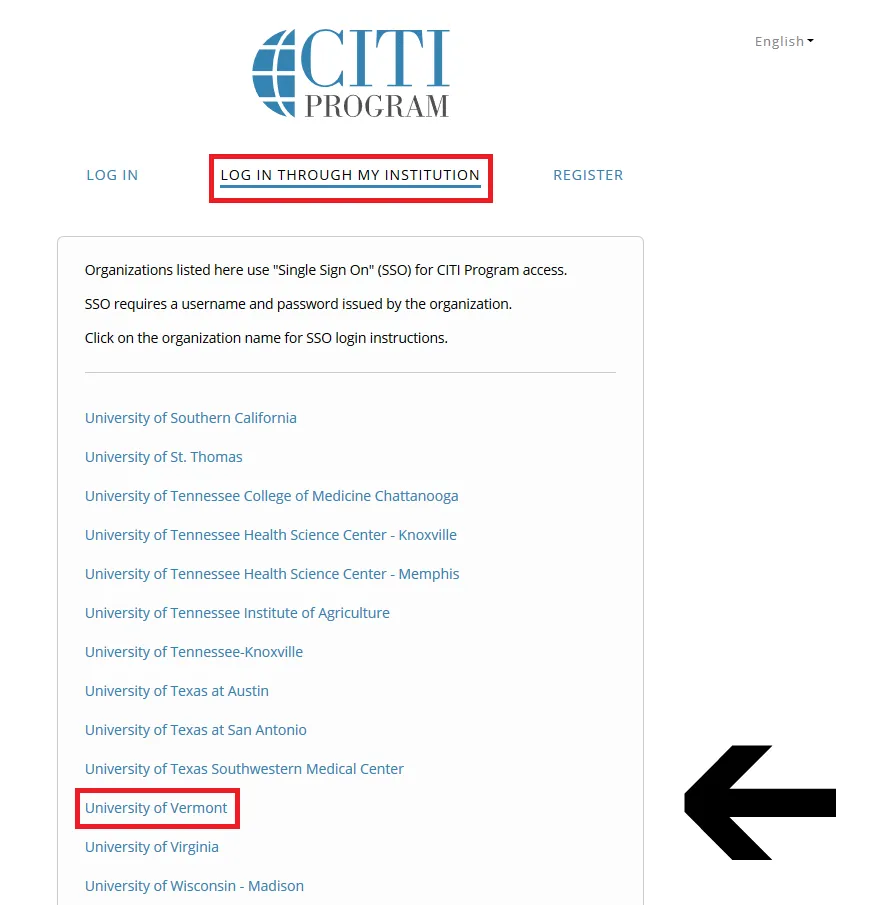
and use your UVM Net ID and password to sign in.

If you receive an error message at sign in, then please refer to instructions on
If you haven’t associated your UVM account with CITI, you will be prompted to choose whether you already have a CITI Program account or if you don't have a CITI Program account.
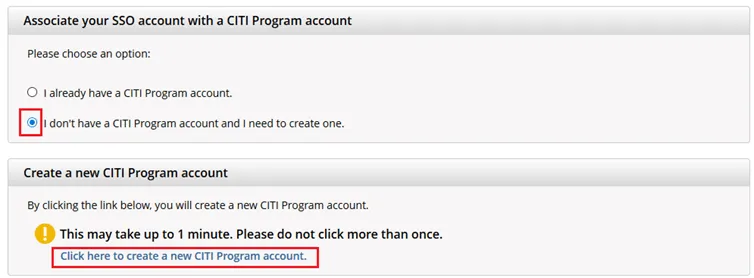
- If you have set up a CITI account in the past, choose the first option
"I already have a CITI Program account" and follw the instructions to link your UVM account. - Otherwise, choose the second option and select
“I don’t have a CITI Program account and I need to create one.” - If you do not receive these prompts, then proceed to step 5.
- If you have set up a CITI account in the past, choose the first option
From the Main Menu, select "View Courses."
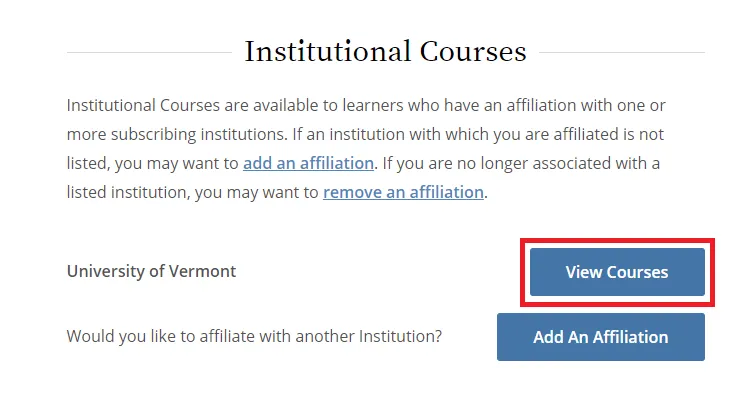
Then, select “Add a Course.”
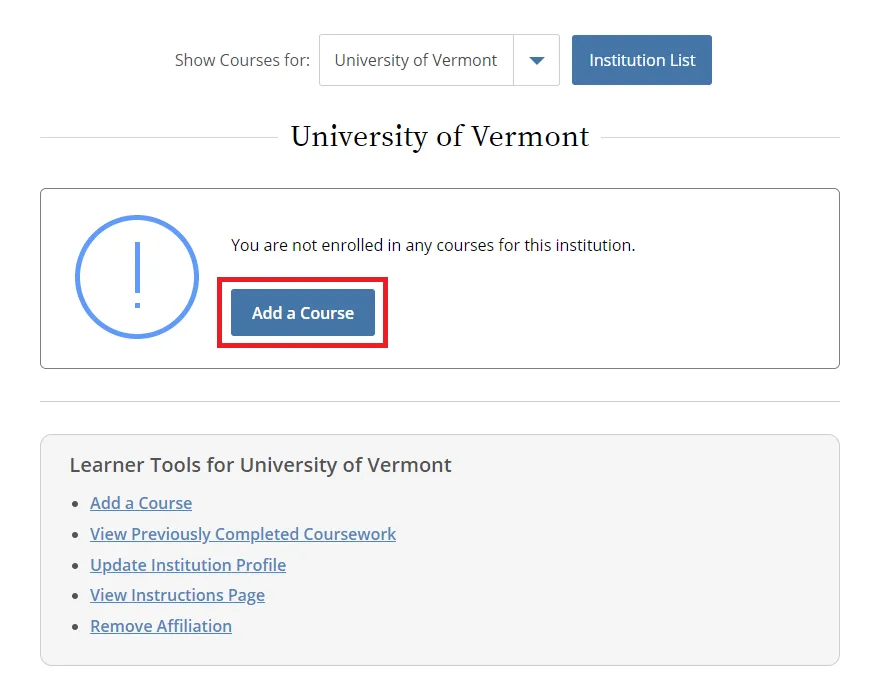
- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC.
- A Guide to Getting Started video is also available.
I would like to register
This allows UVM affiliates to access the CITI training on the same day without a UVM Net ID.
Go to the main CITI Login Page and click the "Register" button at the top right of the page.
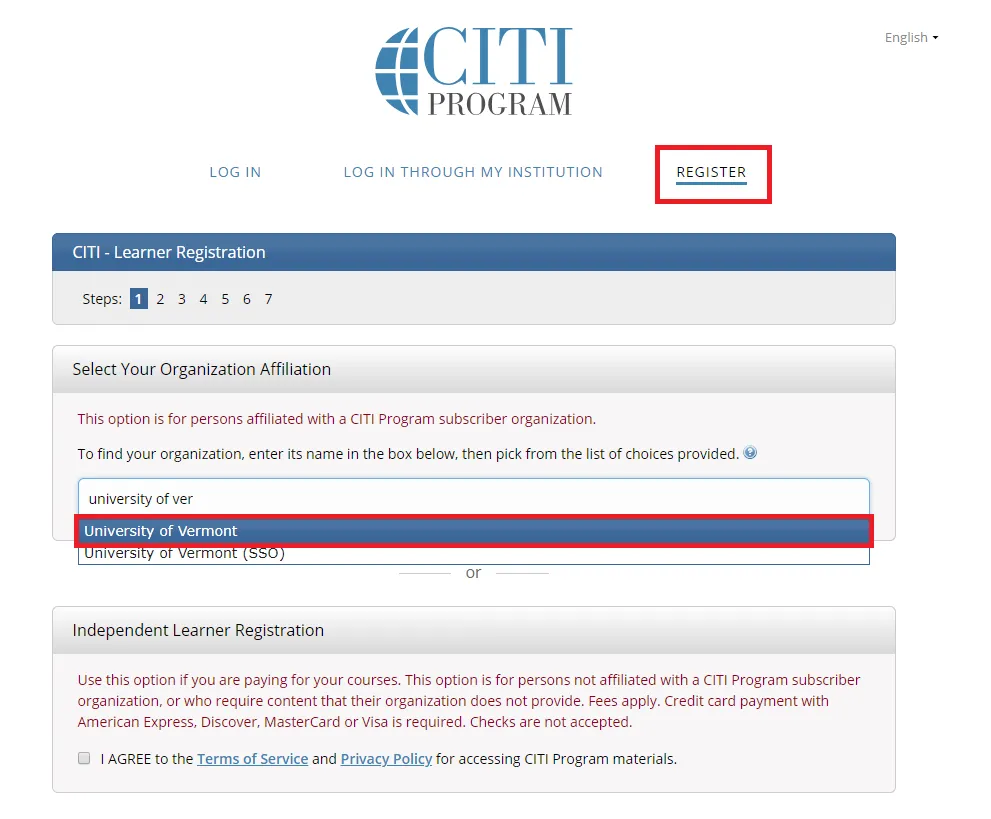
- Step 1: Select Your Organization Affiliation - "University of Vermont".
- Agree to Terms of Service
- Affirm that you are a UVM affiliate
Select "Continue To Create Your CITI Program Username/Password"
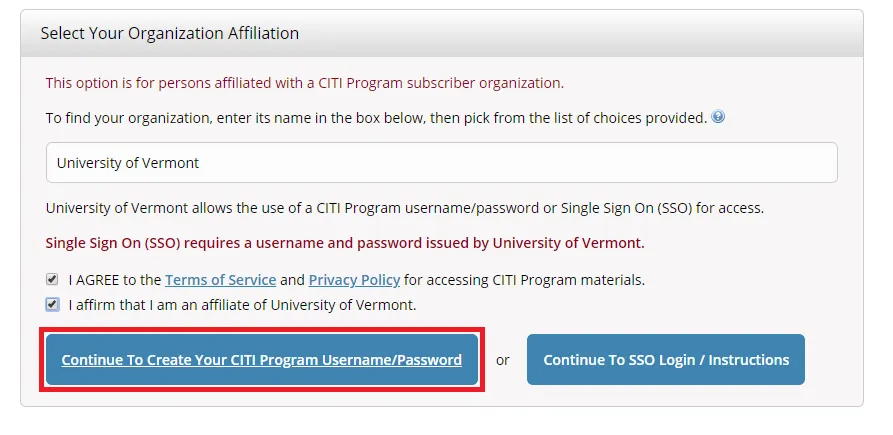
- Complete the Remaining Registration Steps 2 -7.
- Note: Step 5 will ask about receiving Continuing Education Unit credits. Please check "No" to this option and complete the remaining registration steps.
Once you Register through CITI, then you may open the browser and go to the CITI Login Page.
Click “Log In” which is located at the top right of the CITI page.

Then, enter the Username and Password for the CITI Program account you just created and click the blue "Log In" button.

From the Main Menu, select "View Courses."
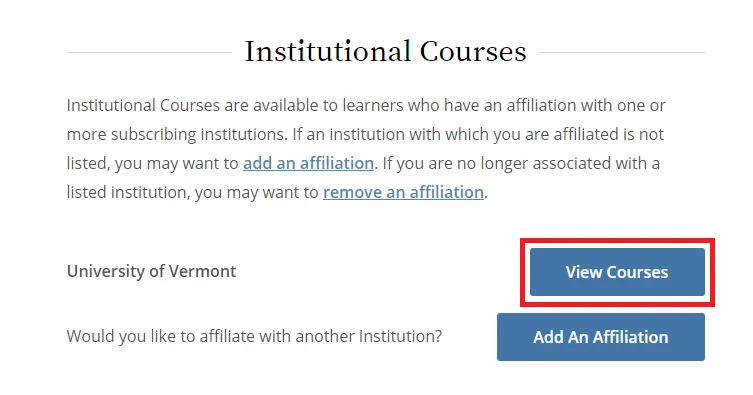
Then, select “Add a Course.”
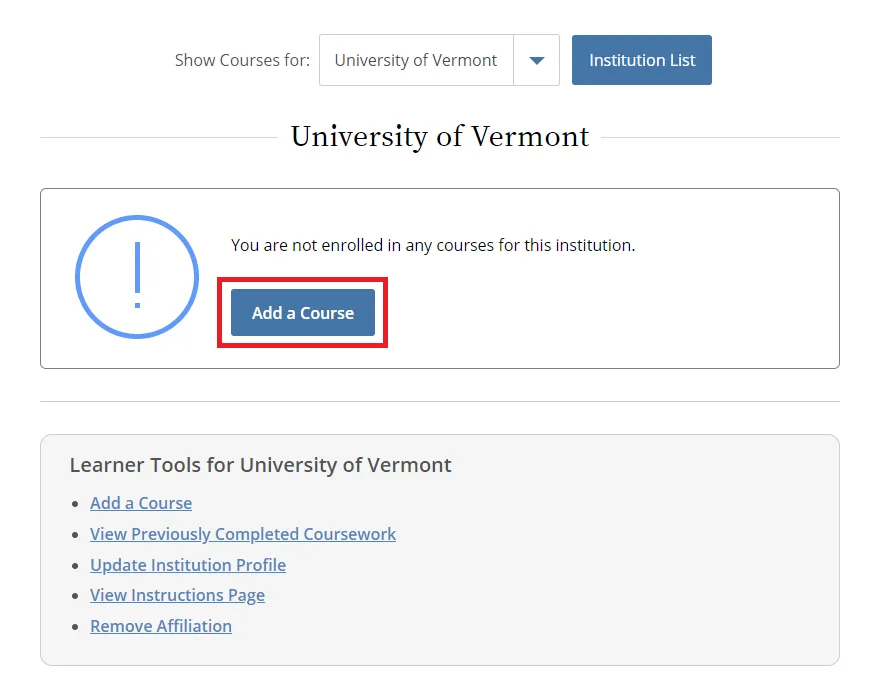
- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC. A Guide to Getting Started video is also available.
Step 2: What training am I required to take and how do I add the course?
Human Subjects Training (IRB)
After selecting "Add a Course" from the CITI Main Menu, follow the table and written instructions below to add the appropriate IRB course(s):
| University of Vermont - CITI Program course(s) | Notes |
|---|---|
| IRB - Human Subjects | Take one of the following to meet the training requirements for Human Subjects Research: -Medical researchers should take Biomedical Research -Social or Behavioral researchers should take Social Behavioral Education Sciences |
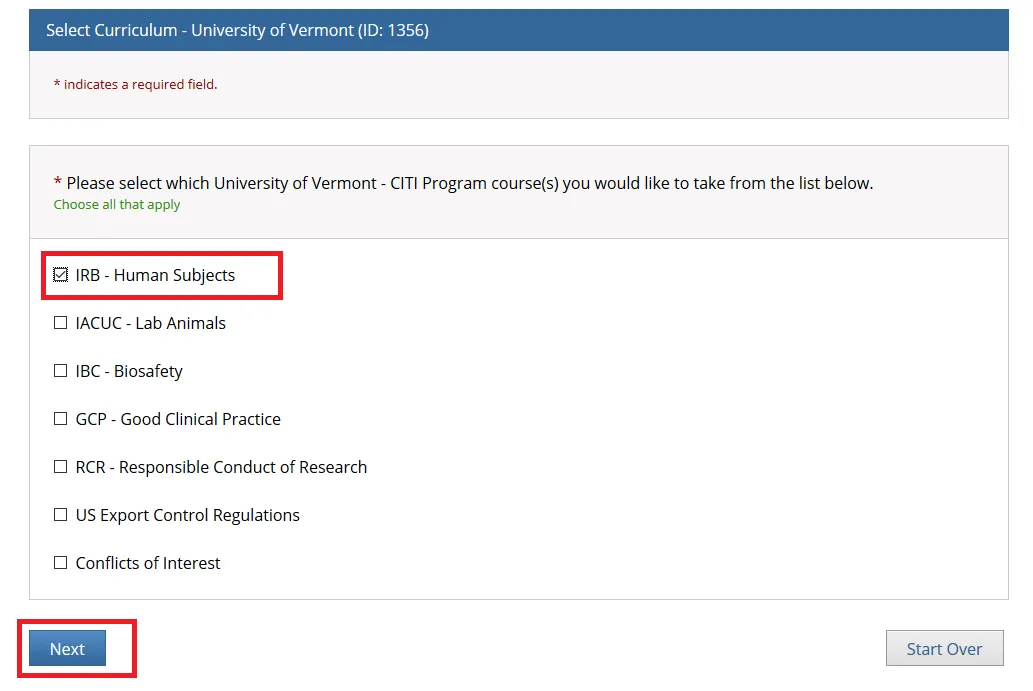
- Check "IRB - Human Subjects" if you are a PI or Research Personnel and click Next.
Note: The following individuals will need to complete both the "IRB - Human Subjects" and "GCP - Good Clinical Practice" training through CITI:
Principal Investigators or Key Personnel on a Clinical Trial involving Human Subjects
All Personnel Affiliated with the UVM Larner College of Medicine
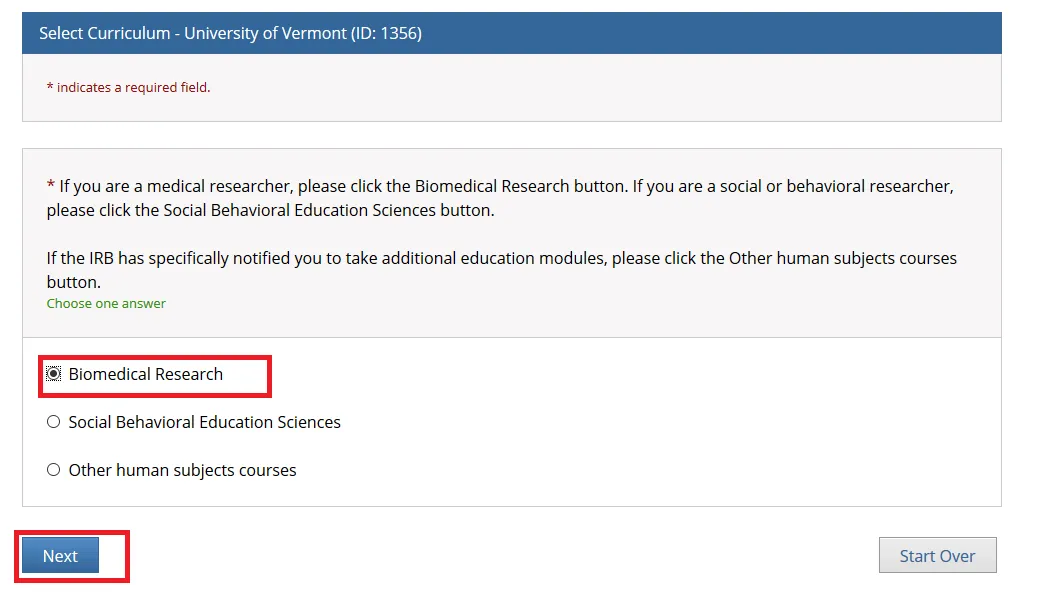
Select one option and click Next. Either the “Biomedical Research” or “Social Behavioral Education Sciences” option will fulfill the UVM Human Subjects training requirement.
If you are a medical researcher, please click the Biomedical Research button.
If you are a social or behavioral researcher, please click the Social Behavioral Education Sciences button.
If the IRB has specifically notified you to take additional education modules, please click the Other human subjects courses button.
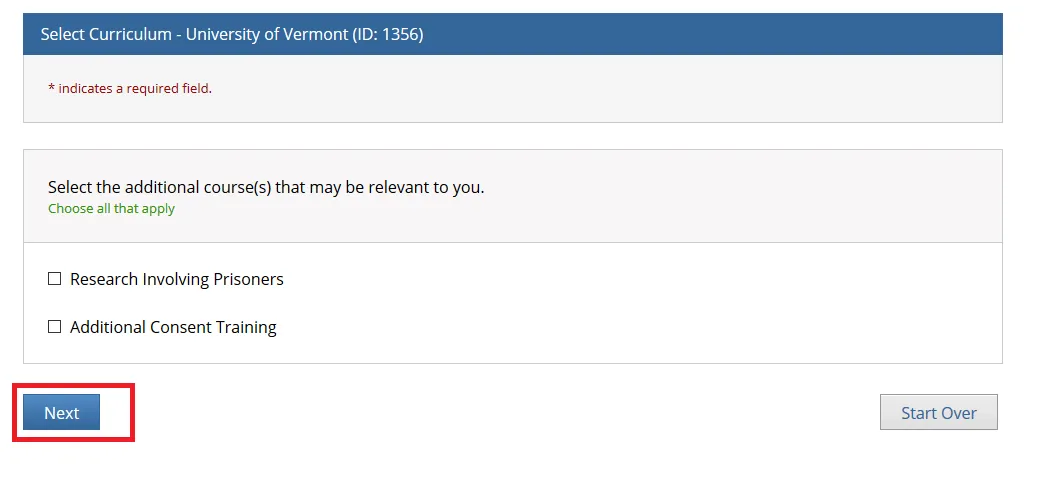
Select the additional course(s) that may be relevant to you and click Next.
Note: You may leave the additional course options unchecked if none apply and simply click "Next" to continue.
The "Research Involving Prisoners" course is required for all personnel conducting research that primarily studies a prisoner population.
The "Additional Consent Training" is optional.
The "Research Involving Prisoners" and "Additional Consent Training" alone Do Not meet all the requirements for human subjects research. You will still need to complete either the “Biomedical Research” or “Social Behavioral Education Sciences” course.
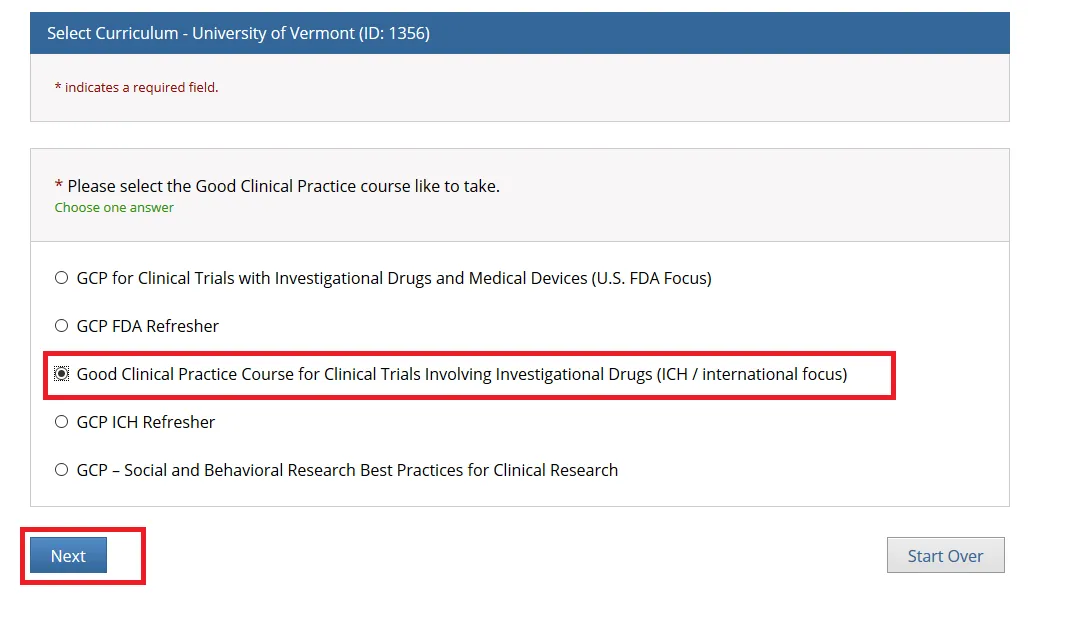
Then, choose ONE “Good Clinical Practice” course if on a Clinical Trial or a UVM Larner College of Medicine Affiliate and click Next.
Note: When you select the “Good Clinical Practice Course for Clinical Trials Involving Investigational Drugs (ICH / international focus)” this is the same as “GCP for Clinical Trials with Investigational Drugs and Biologics (ICH Focus)” and the course does include the Biologics component.
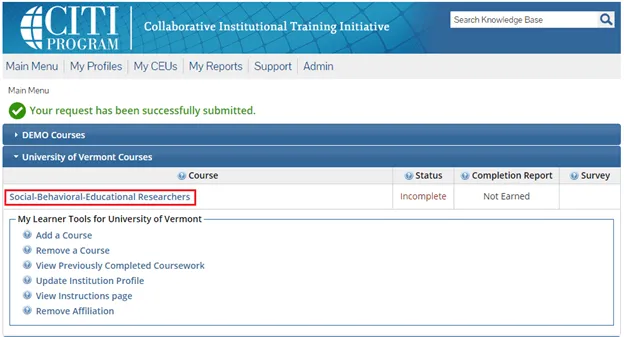
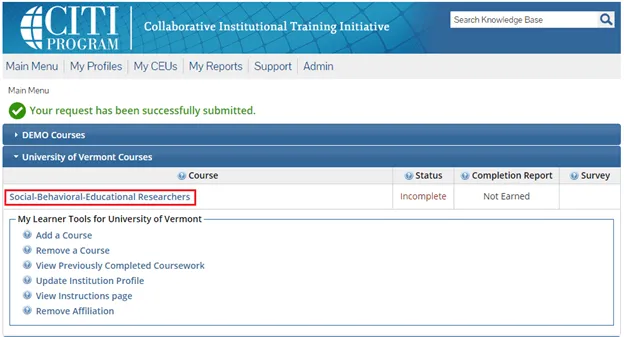
The course(s) will now be added to your UVM course listing! Click on the newly added course within “University of Vermont Courses” to begin the training.
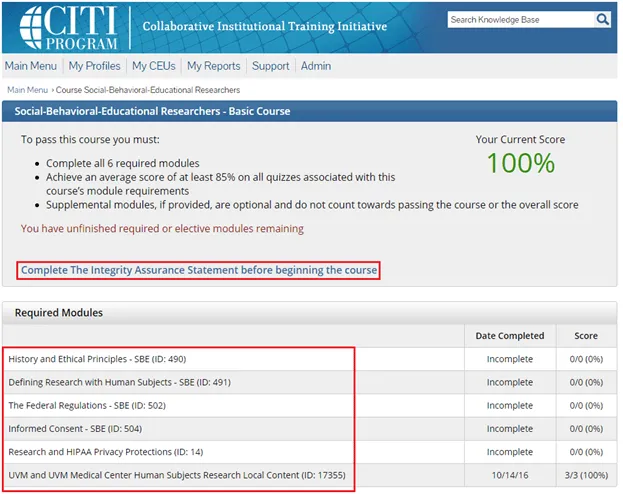
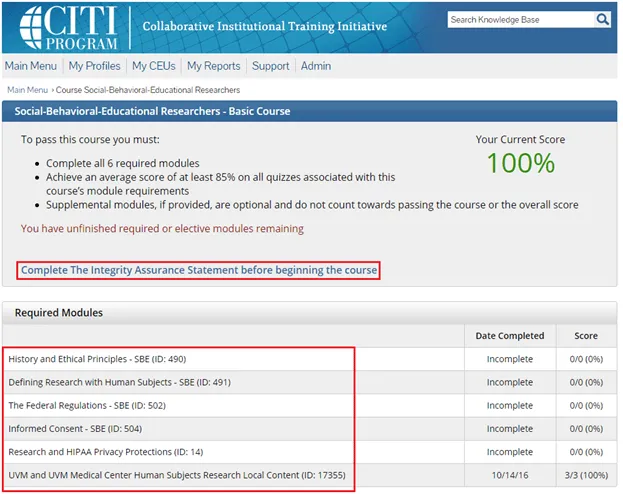
Complete the Integrity Assurance Statement and then begin working through the course modules.
Note: You only need to take the modules listed under “Required Modules” to complete the course and that supplemental modules may be available to you as a member of the UVM research community.
A record of the training completion can be found on our IRB Tutorial Completion page.
Make sure you completed the appropriate course. See the Human Subjects Training section of our Frequently Asked Questions page for further details.
The completion list updates every morning, so if you just completed the course today, please wait a day to find your name in the dropdown.
Good Clinical Practice Training (GCP)
After selecting "Add a Course" from the CITI Main Menu, follow the table and written instructions below to add the appropriate course:
| University of Vermont - CITI Program course(s) | Notes |
|---|---|
| GCP - Good Clinical Practice | Take one of the following if on a Clinical Trial or a UVM Larner College of Medicine Affiliate: U.S. FDA Focus -GCP for Clinical Trials with Investigational Drugs and Medical Devices OR ICH Focus - applicable to those engaged in international research -GCP for Clinical Trials with Investigational Drugs and Biologics OR Social and Behavioral Research -GCP – Social and Behavioral Research Best Practices for Clinical Research |
Check “IRB – Human Subjects” and "GCP – Good Clinical Practice" if you are a PI or Research Personnel on a Clinical Trial involving Human Subjects or a UVM Larner College of Medicine Affiliate. All those affiliated with the UVM Larner College of Medicine are required to complete GCP training regardless of whether the research project fits the NIH clinical trial definition.
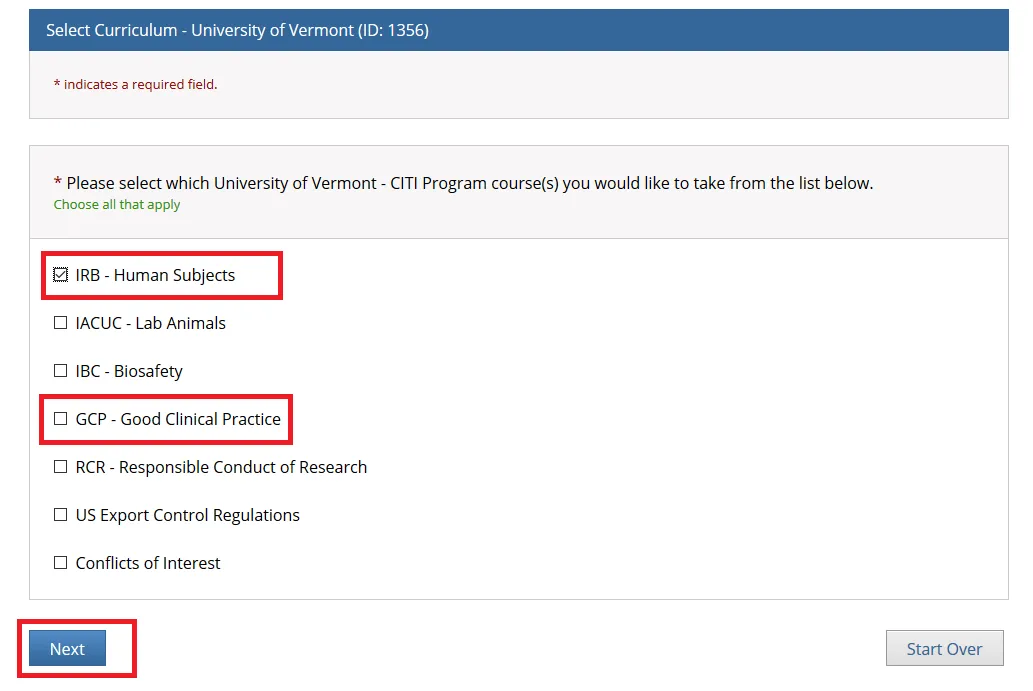
- Select one option and click Next. Either the “Biomedical Research” or “Social Behavioral Education Sciences” option will fulfill the UVM Human Subjects training requirement.
If you are a medical researcher, please click the Biomedical Research button.
If you are a social or behavioral researcher, please click the Social Behavioral Education Sciences button.
If the IRB has specifically notified you to take additional education modules, please click the Other human subjects courses button.
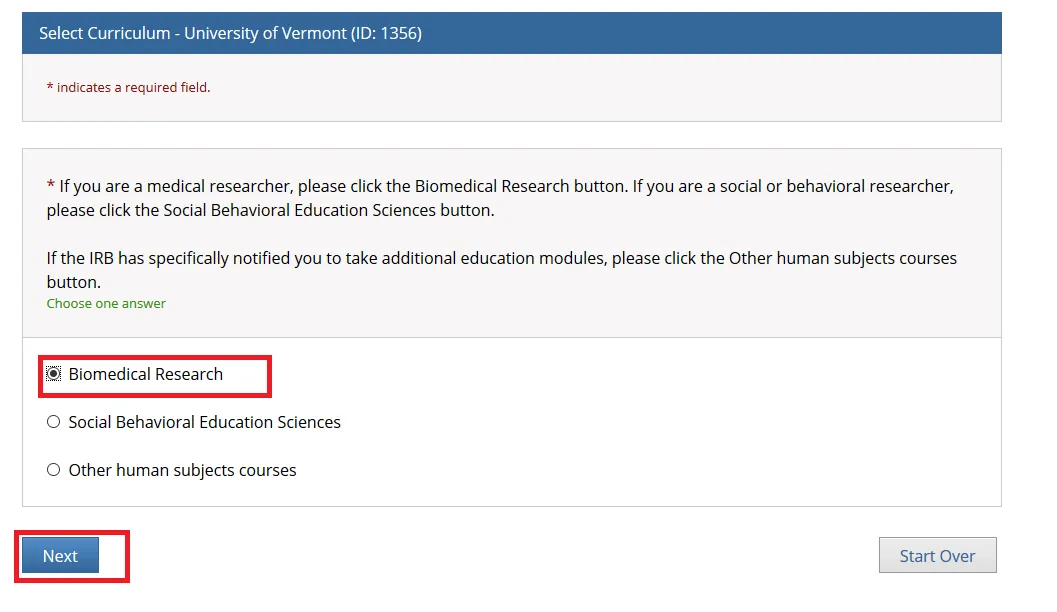
Select the additional course(s) that may be relevant to you and click Next.
Note: You may leave the additional course options unchecked if none apply and simply click "Next" to continue.
The "Research Involving Prisoners" course is required for all personnel conducting research that primarily studies a prisoner population.
The "Additional Consent Training" is optional.
The "Research Involving Prisoners" and "Additional Consent Training" alone Do Not meet all the requirements for human subjects research. You will still need to complete either the “Biomedical Research” or “Social Behavioral Education Sciences” course.
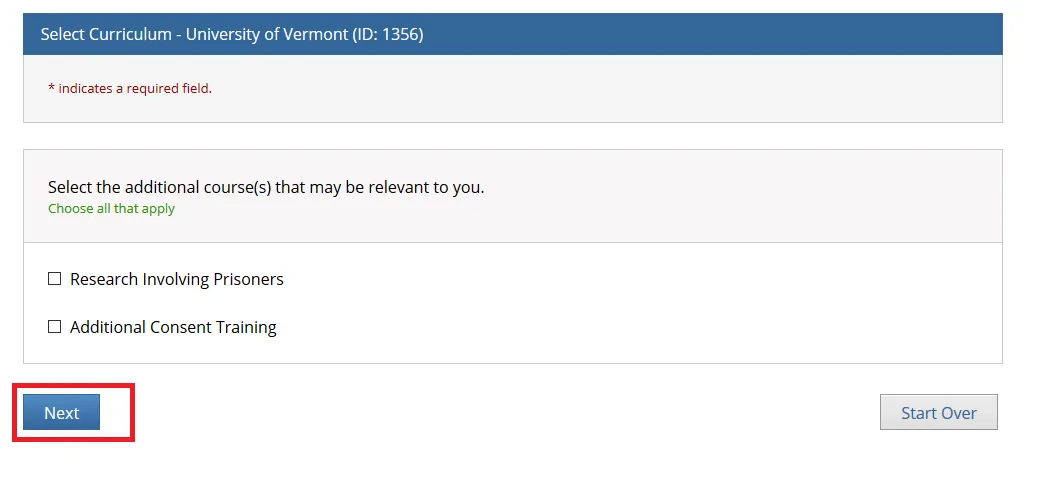
Then, choose ONE “Good Clinical Practice” course if on a Clinical Trial or a UVM Larner College of Medicine Affiliate and click Next.
Note: When you select the “Good Clinical Practice Course for Clinical Trials Involving Investigational Drugs (ICH / international focus)” this is the same as “GCP for Clinical Trials with Investigational Drugs and Biologics (ICH Focus)” and the course does include the Biologics component.
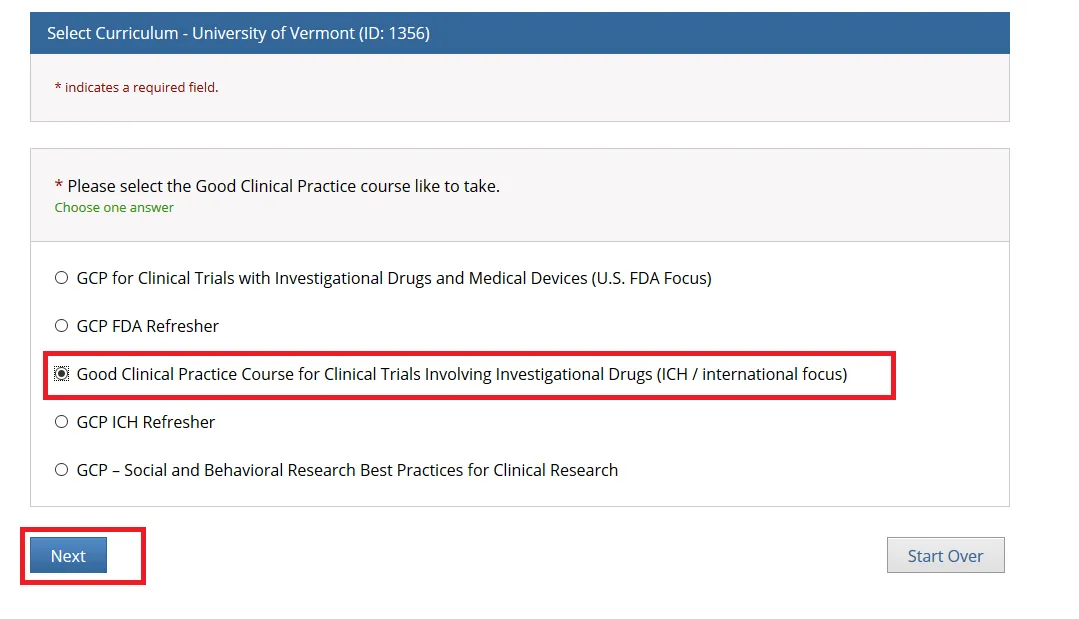
The course(s) will now be added to your UVM course listing! Click on the newly added course within “University of Vermont Courses” to begin the training.
Complete the Integrity Assurance Statement and then begin working through the course modules.
Note: You only need to take the modules listed under “Required Modules” to complete the course and that supplemental modules may be available to you as a member of the UVM research community.
A record of the training completion can be found on our IRB Tutorial Completion page.
Make sure you completed the appropriate course. See the Good Clinical Practice Training section of our Frequently Asked Questions page for further details.
The completion list updates every morning, so if you just completed the course today, please wait a day to find your name in the dropdown.
Biosafety Research Training (IBC)
After selecting "Add a Course" from the CITI Main Menu, follow the table and written instructions below to add the appropriate IBC course(s):
| University of Vermont - CITI Program course(s) | Notes |
|---|---|
| IBC - Biosafety | CITI Requirements: -Depending on the level of containment, you will need to complete either the BSL-1 or BSL-2 Basic Course. The BSL-2 Basic Course meets all requirements for BSL-1 related work. AND other CITI course(s) if applicable: -OSHA Bloodborne Pathogens (check with EHS at safety@uvm.edu to see if this is required) -Animal Biosafety -Select Agents/DURC -Nanotechnology |
Check “IBC - Biosafety” if you are PI or Research Personnel and click Next.
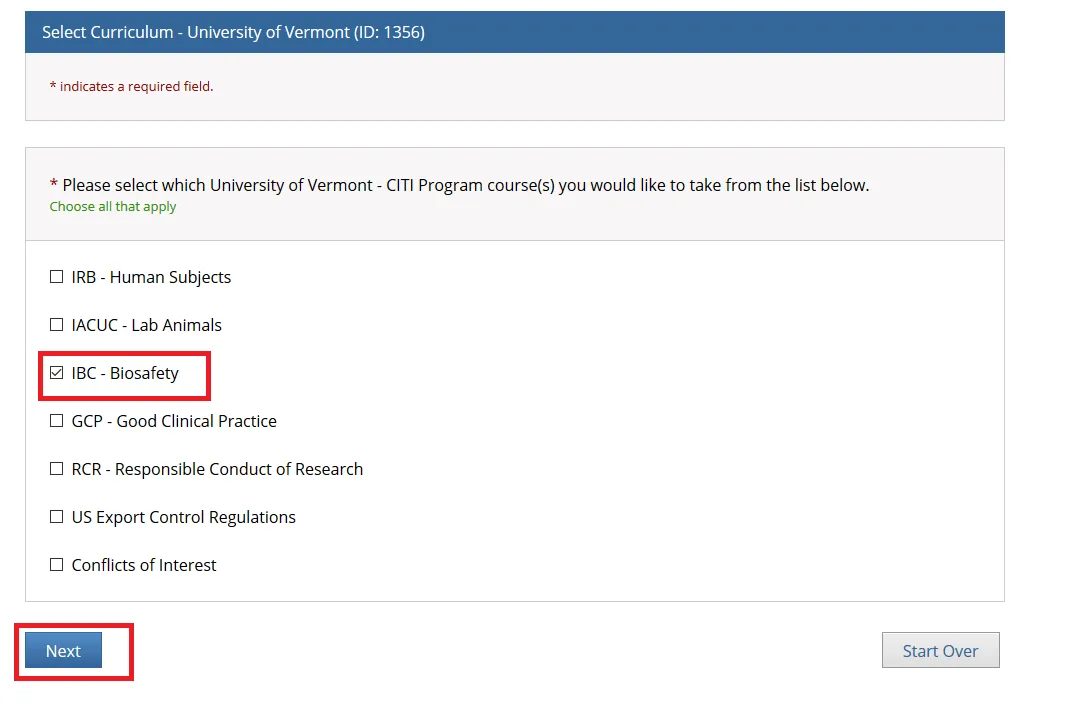
"Do you want to take the BSL-1 or BSL-2 Basic Course?"
Choose the applicable option and click Next.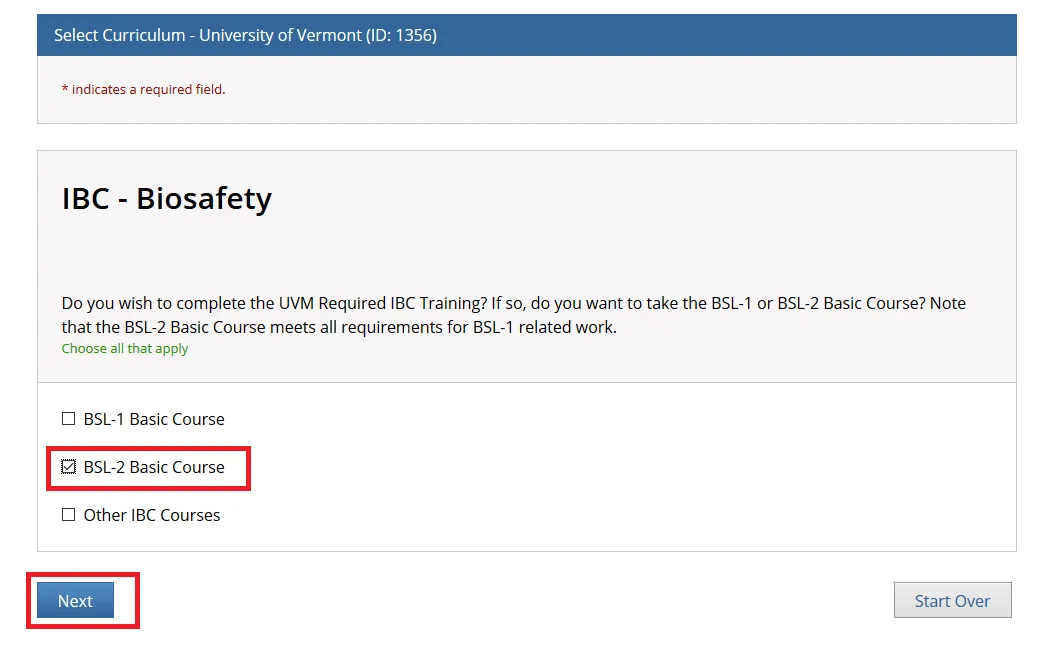
"Are you currently or do you anticipate conducting research that includes exposure to bloodborne pathogens?"
Answer as applicable and click Next.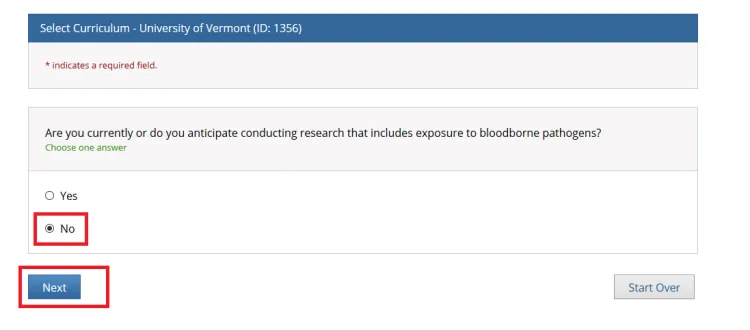
"Are you currently or do you anticipate conducting research that uses Select Agents?"
Answer as applicable and click Next.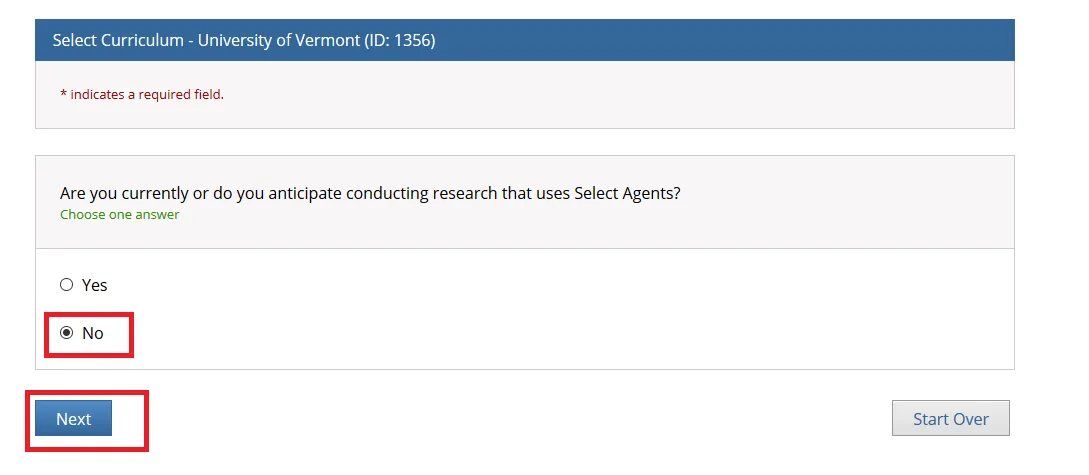
"Are you currently or do you anticipate conducting IBC research that includes animal research?"
Answer as applicable and click Next. If you check "Yes", then the "Animal Biosafety" course will be added to your UVM course listing.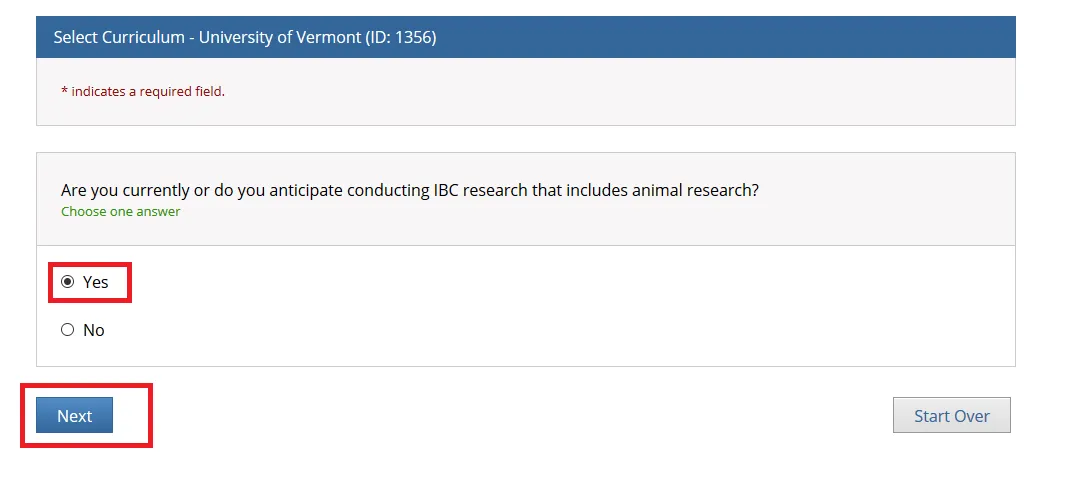
"Are you currently or do you anticipate conducting research that uses nanotechnology?"
Answer as applicable and click Next.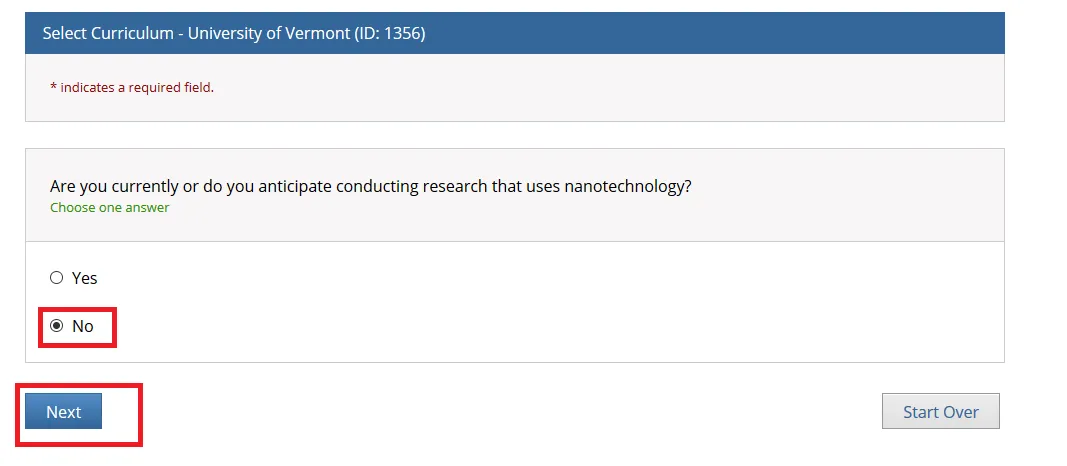
The course(s) will now be added to your UVM course listing! Click on the newly added course within “University of Vermont Courses” to begin the training.
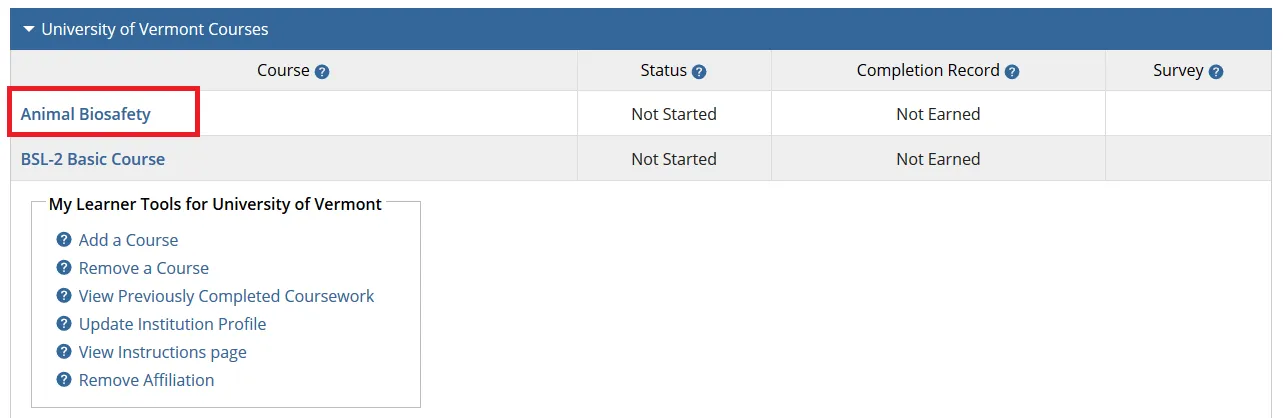
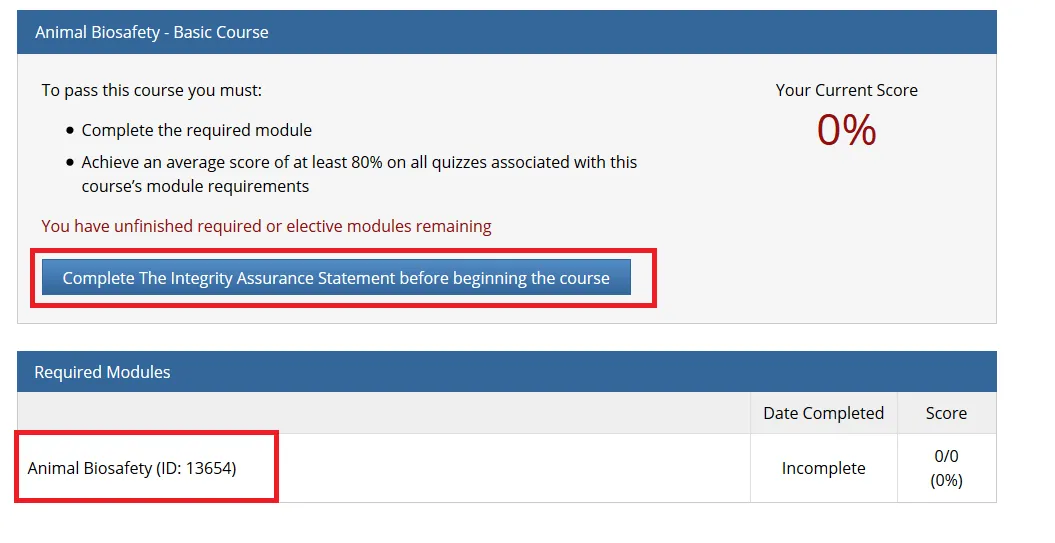
Complete the Integrity Assurance Statement and then begin working through the course modules.
Note: You only need to take the modules listed under “Required Modules” to complete the course and that supplemental modules may be available to you as a member of the UVM research community.
- A record of the training completion can be found on our IBC Tutorial Completion Page.
Make sure you completed the appropriate course. See the Biosafety Research Training section of our Frequently Asked Questions page for further details.
The completion list updates every morning, so if you just completed the course today, please wait a day to find your name in the dropdown.
Laboratory Animals Training (IACUC)
After selecting "Add a Course" from the CITI Main Menu, follow the table and written instructions below to add the appropriate IACUC course(s):
| University of Vermont - CITI Program course(s) | Notes |
|---|---|
| IACUC - Lab Animals | CITI Requirements: -Take the General Lab Animal Training course in addition to any Animal-Specific Courses (Amphibians, Cattle, Fish, etc.) Occupational Health and Safety Program for Employees Working with Animals: -All personnel working with animals are required to complete a baseline risk assessment form prior to working with animals. Subsequent health reviews are required annually. |
Check “IACUC - Lab Animals” if you are PI or Research Personnel and click Next.
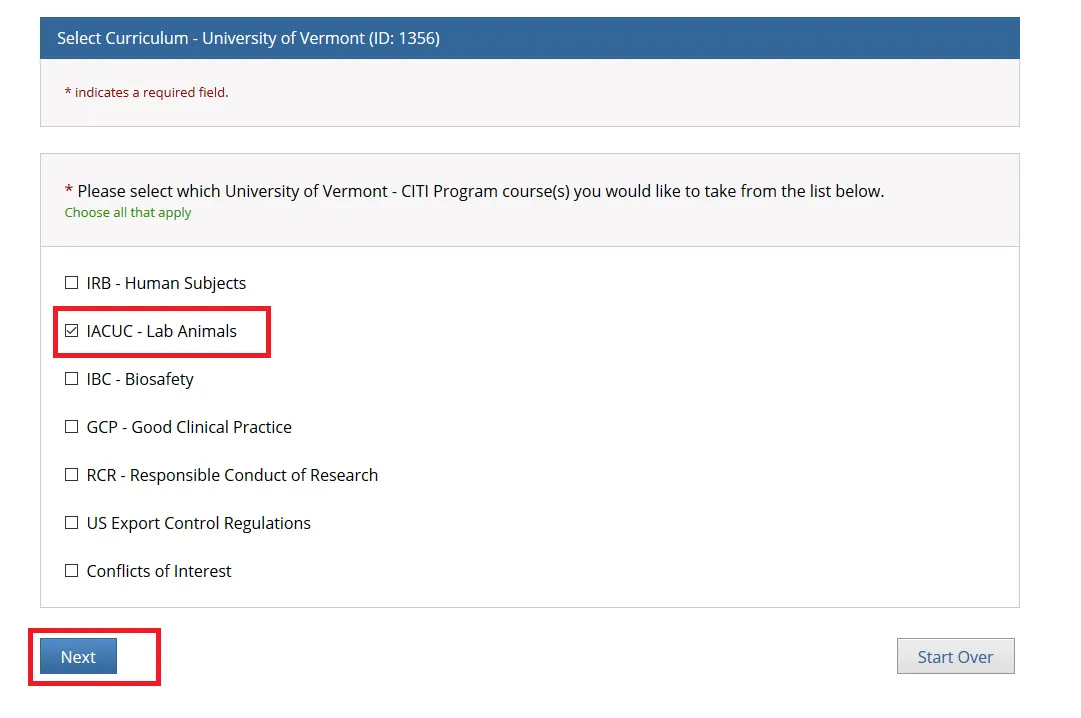
Choose the "General Lab Animal Training" option and click Next.
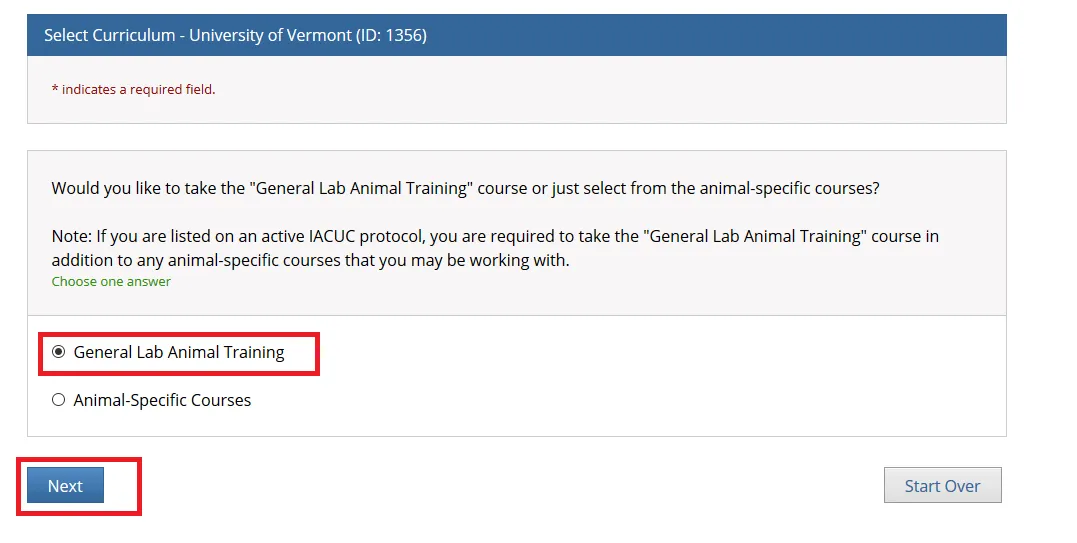
Select from the list of "Animal-Specific Courses" that may be relevant to you and click Next.
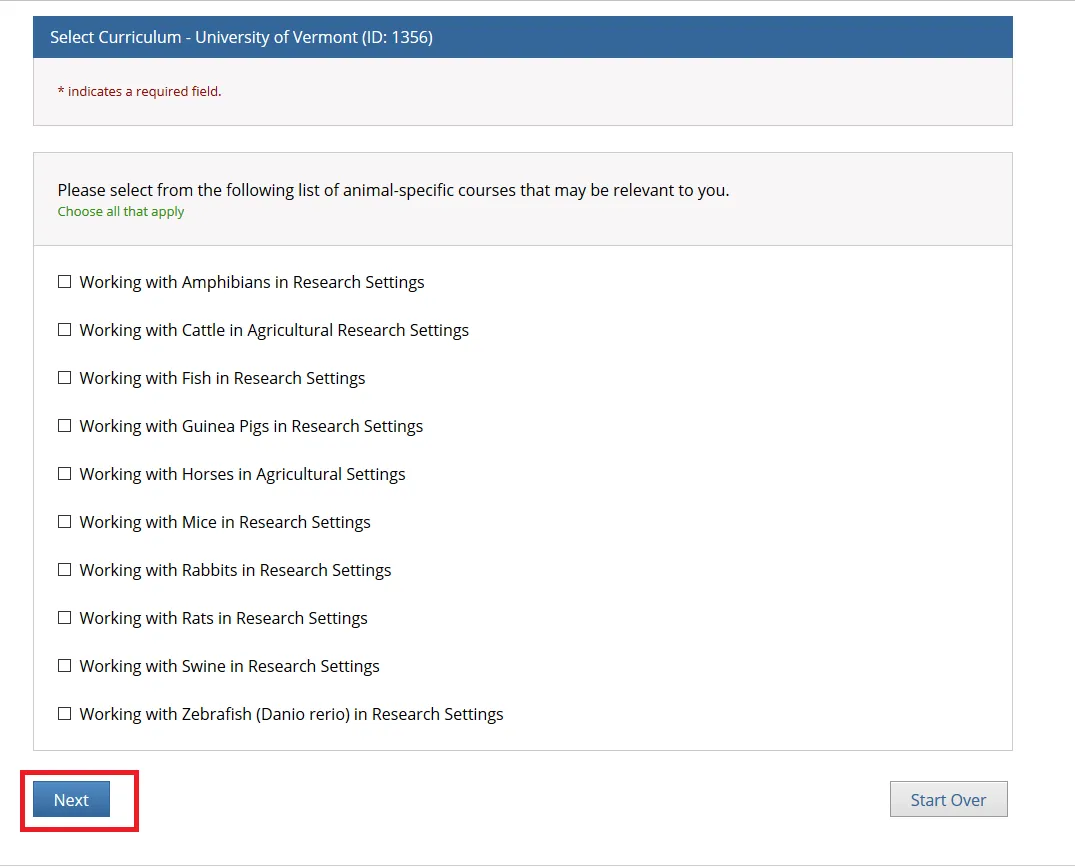
The course(s) will now be added to your UVM course listing! Click on the newly added course within “University of Vermont Courses” to begin the training.
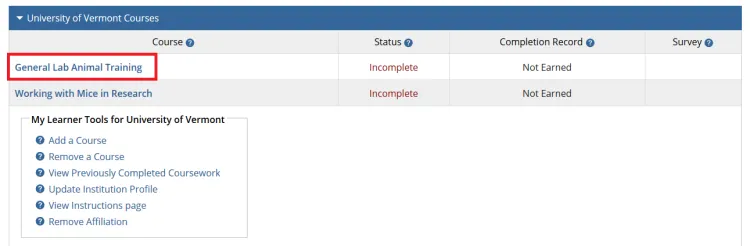
Complete the Integrity Assurance Statement and then begin working through the course modules.
Note: You only need to take the modules listed under “Required Modules” to complete the course and that supplemental modules may be available to you as a member of the UVM research community.
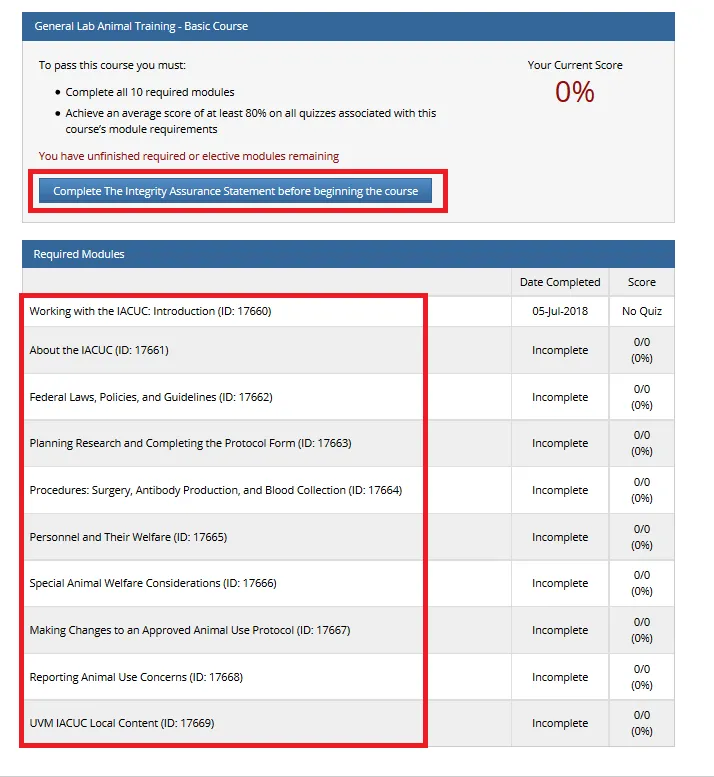
- A record of the training completion can be found on our IACUC Tutorial Completion page (requires UVM Net ID login).
Make sure you completed the appropriate course. See the Laboratory Animals Training section of our Frequently Asked Questions page for further details.
The completion list updates every morning, so if you just completed the course today, please wait a day to find your name in the dropdown.
(IACUC) Occupational Health and Safety Program
General Information
- An occupational health and safety program is a required part of the overall animal care and use program.
- The focus is maintaining a safe and healthy workplace.
- UVM's Department of Risk Management has contracted with two medical facilities to provide occupational health monitoring for all UVM personnel who work with animals. Champlain Medical, is located in South Burlington, 802-448-9370. Additionally, all UVM students completing the questionnaire to be added as key personnel will have questionnaires forwarded to UVM Student Health Services.
- All personnel working with animals are required to complete a form or the declination located on the form prior to working with animals.
- Subsequent health reviews are required triennially by submitting an updated form. If, however, you have health concerns prior to any review you should contact Champlain Medical immediately.
- As the assessments are received at Champlain Medical or UVM Student Health Services, each person’s responses are evaluated. Those persons who are designated to be at risk for work-related disease will be contacted to make an appointment for a physical examination.
Animal Handler Occupational Health Questionnaire
If you are an EMPLOYEE:
The Animal Handler Occupational Health Questionnaire can be accessed by following this link to the EMPLOYEE ONLY form.
If you are a STUDENT:
The Animal Handler Occupational Health Questionnaire can be accessed by following these instructions for the STUDENT ONLY form.
Troubleshooting
Make sure you have a UVM Net ID
Request for UVM Net ID form (DOCX)
The UVM Network ID should be 8 characters or less.
- If you haven’t updated your password in the past year, you may need to go to UVM's Account Management page to change your Net ID password or reset a forgotten password.
- Please note that if you went through our "Request for UVM Net ID" process, you will need to reference your PIN when resetting your Net ID password. This PIN is issued when the Request for Net ID form is first processed. If you have lost your PIN, please contact rpo@uvm.edu.
Associate your UVM Net ID with CITI
Once you have an active UVM Net ID, then you may open the browser and go to the CITI Login Page.
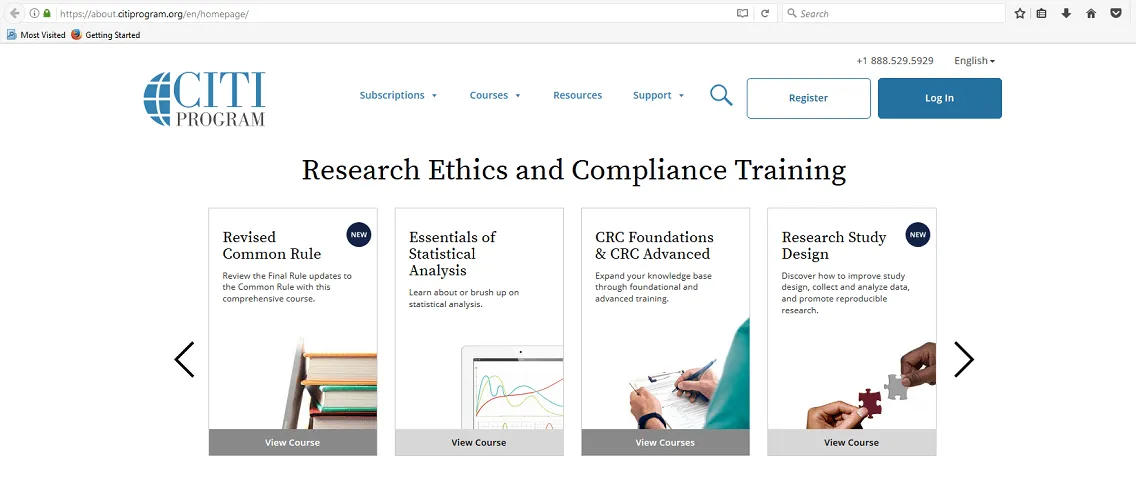
Click “Log In” which is located at the top right of the CITI page.

Then, click "Log In Through My Institution" to view the organizations listed to use "Single Sign On" (SSO) for CITI Program access, scroll down to click on “University of Vermont”...
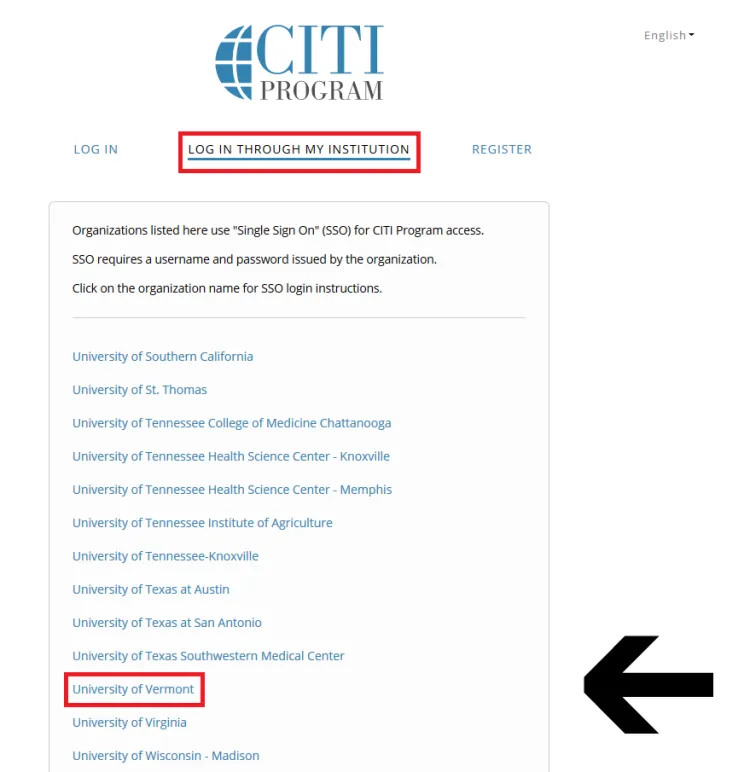
and use your UVM Net ID and password to sign in.

If you haven’t associated your UVM account with CITI, you will be prompted to choose whether you already have a CITI Program account or if you don't have a CITI Program account.
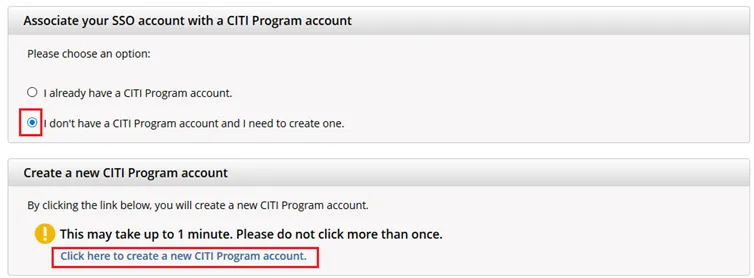
- If you have set up a CITI account in the past, choose the first option
"I already have a CITI Program account" and follow the instructions to link your UVM account. - Otherwise, choose the second option and select
“I don’t have a CITI Program account and I need to create one.” - If you do not receive these prompts, then please contact CITI Support.
Create a separate account through CITI (Register)
This allows UVM affiliates to access the CITI training without a UVM Net ID.
Go to the main CITI Login Page and click the "Register" button at the top right of the page.
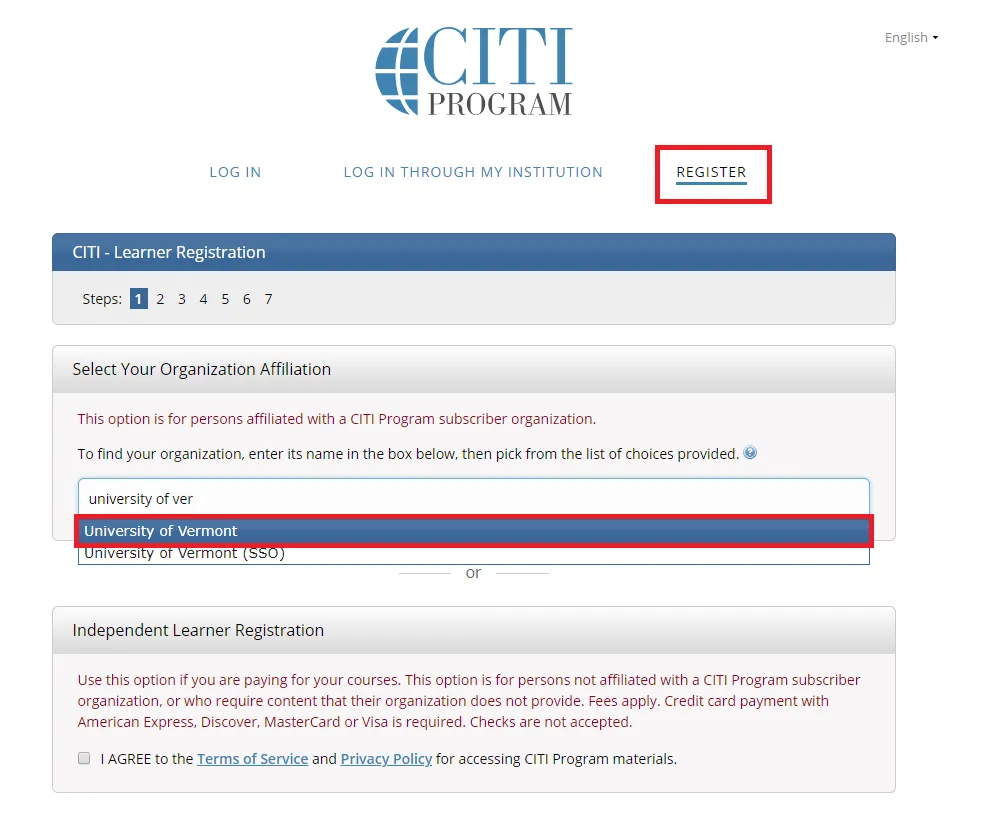
- Step 1: Select Your Organization Affiliation - "University of Vermont".
- Agree to Terms of Service
- Affirm that you are a UVM affiliate
Select "Continue To Create Your CITI Program Username/Password"
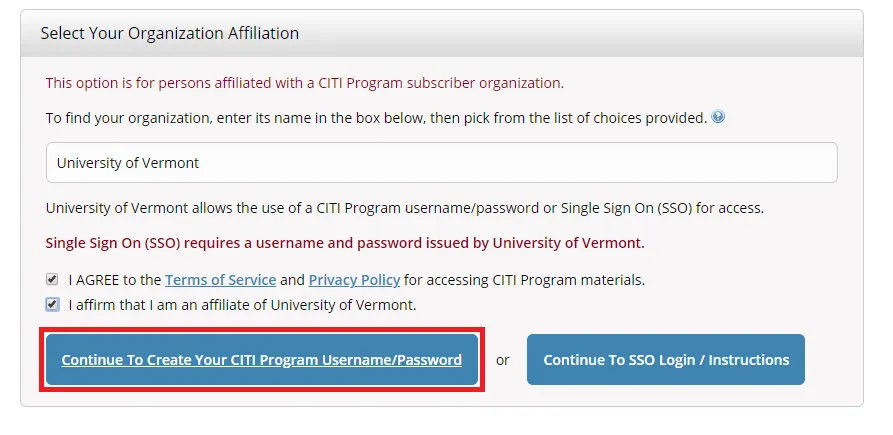
- Complete the Remaining Registration Steps 2-7.
- Note: Step 5 will ask about receiving Continuing Education Unit credits. Please check "No" to this option and complete the remaining registration steps.
Merge two CITI Program accounts
Please contact CITI Support to merge duplicate accounts. You may also visit the CITI Program Support Center for assistance.