AAALAC Site Visit!
UVM has begun the process of renewing its AAALAC accreditation. AAALAC Accreditation demonstrates to granting agencies, other sources of extramural funding and to the public that the University maintains a high standard of care for its research animals. UVM has been fully accredited for over 25 years. The initial step in the process is submission of a detailed description of UVM’s program of animal care; after that is evaluated, site visitors from AAALAC will perform an on-site evaluation of our program and facilities. This site visit will be scheduled for the first quarter of 2025. Since the site visitors request to see laboratories and meet with investigators, we will give you as much notice as possible with regards to their schedule.
Abbey Dattilio, IACUC’s Monitoring and Compliance Specialist, has already begun working with researchers to ensure their aeas are prepared for the visit. If you would like assistance, please email us at iacuc@uvm.edu
Emergency Response Plan
To ensure the health and well-being of animals during emergencies, the Office of Animal Care Management (OACM) has an animal response plan in place titled the Office of Animal Care Management and Satellite Animal Facilities Emergency Response Plan. This plan aligns with the standards for emergency preparedness outlined in the Animal Welfare Act and the Guide for Care and Use of Laboratory Animals and serves as a supplement to UVM’s comprehensive Emergency Management and Continuity of Operations plan. The animal response plan undergoes annual review, with essential personnel receiving annual refresher training to include updates.
“Essential personnel” outlined in the plan include OACM’s veterinary and husbandry staff, the facility manager, managers of designated satellite facilities, and all personnel responsible for animal care in those areas. While research staff are not tasked with plan activation during emergencies, it is important for you to understand the plan for animal care in various emergency scenarios.
It is especially important that study teams located in satellite facilities understand their role and responsibilities in the event of an emergency as OACM husbandry staff are not present to assist. Careful review and training of the satellite study team members is very important. Please reach out with any questions about the plan and if you have specific requirements for your animals during emergencies, please contact IACUC or OACM staff.
New Drupal Site
Have you had a chance to check out the new Research Protections Office website?! All the RPO committee pages moved from the Drupal 7 platform to the new Drupal 10 in August. Updates include UVM’s new design, logos and iPhone friendly user interface. Many links have changed and content has been moved so you will have to create new bookmarks if you have previously saved IACUC pages to your favorites tab.
Click Tips!
There are some upcoming updates to the UVMClick-IACUC eforms that we wanted to bring your attention.
The Protocol Team Member page as of October 30th, will have a new question to capture conflicts of interest. This is a mandatory question for each team member going forward. For existing protocols, you will be prompted to answer this question for all current team members at the time of your next amendment or triennial review, whichever comes first. Help text will be included next to the question within UVMClick and will reference the University of Vermont Policy - Disclosing your significant financial Interest (SFI) with the definition of significant financial interest.
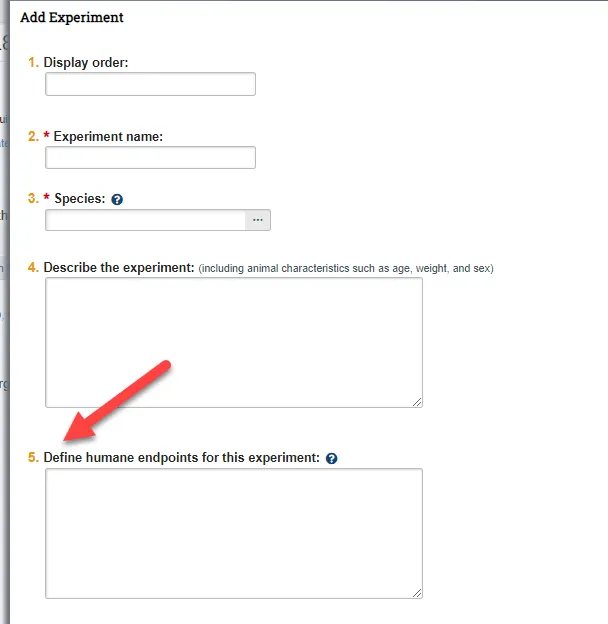
The “Add Experiment” pop up on the Experiments page has a new field for investigators to describe humane endpoints. We ask that researchers continue to define endpoints within the individual procedure eforms along with your monitoring plans. IACUC will be updating the language of this new question to be more clear in our November updates.
Policy Updates
Many of the policies and procedures manual section updates were non-substantive. However, below is a list of revised sections that may impact your research. You can find the full version of the manual here.
4.2 Projects Utilizing Hazards in Animals – Now requires the upload of Safety Data Sheets (SDSs) to the Substance eform when adding a hazardous substance to your protocol. If you are not sure whether a substance is hazardous or not, look for the Globally Harmonized System (GHS) classification on the SDS form. Having this information at time of IACUC review will streamline the review process.
Example:

- 6.9 Standard Procedures and Substances – Minor editorial changes
- 7.3 Investigator Training Requirements – Minor editorial changes
- 7.4 Occupational Health and Safety – Minor editorial changes
15.27 Policy Regarding the Use of Random Source Animals for Research – Minor editorial changes