Acid Deposition
National Atmospheric Deposition Program/National Trends Network

The ecological consequences of atmospheric acid deposition have been well studied in the Northeastern US. Through these investigations, observations can be made that acid rain has led to the decline of red spruce in the 1970s and 80s, the leaching loss of calcium and other cations from soil, and the acidification of lakes and streams. Two measures of acid deposition are sulfate (SO4) and nitrate (NO3); when emitted as air pollutants, these molecules can form acids through reactions with water in the atmosphere, creating what we know as ‘acid rain’. Recognizing this serious environmental threat, regulations were enacted to control emissions of sulfur and nitrogen oxides, which react in the atmosphere to produce acidic compounds; as a result, acidic deposition has declined and ecosystem recovery is underway.
The Data
National Atmospheric Deposition Program (NADP) has been monitoring precipitation chemistry in the US since 1978 through the National Trends Network (NTN) program. The 250 national NTN sites collected data on the amounts, trends, and geographic distributions of acids, nutrients, and base cations in precipitation.
NTN sites are predominantly located away from urban areas and point sources of pollution. Each site is equipped with a precipitation chemistry collector and gage. The automated collector ensures that the sample is exposed only during precipitation (wet-only-sampling). Site operators follow standard operational procedures to help ensure NTN data is comparable. All samples are analyzed and verified by the Central Analytical Laboratory (CAL) at the Illinois State Water Survey (ISWS). Measurements include acidity (H+ as pH), conductance, calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), sulfate (SO42-), nitrate (NO3-), chloride (Cl-), and ammonium (NH4+). Deposition is expressed as a concentration of the pollutant, which reflects the amount of water in which it is transported.
The Forest Ecosystem Monitoring Cooperative has conducted atmospheric deposition monitoring for over thirty years at the Proctor Maple Research Center in Underhill. The Underhill NADP/NTN site has been a cornerstone of FEMC monitoring and research, providing key information on the sources of pollution, trends in deposition rates and how this influences forested ecosystems. The continental scale of NTN sites reveals spatial and temporal trends in acid deposition in Vermont and the Northeast and allows comparison with other regions of the U.S. Today, this information is necessary to understand how air quality policies have ameliorated acid deposition across the region, and to inform future policy and management decisions to sustain the health of the region’s forested ecosystems.
2018 in Summary
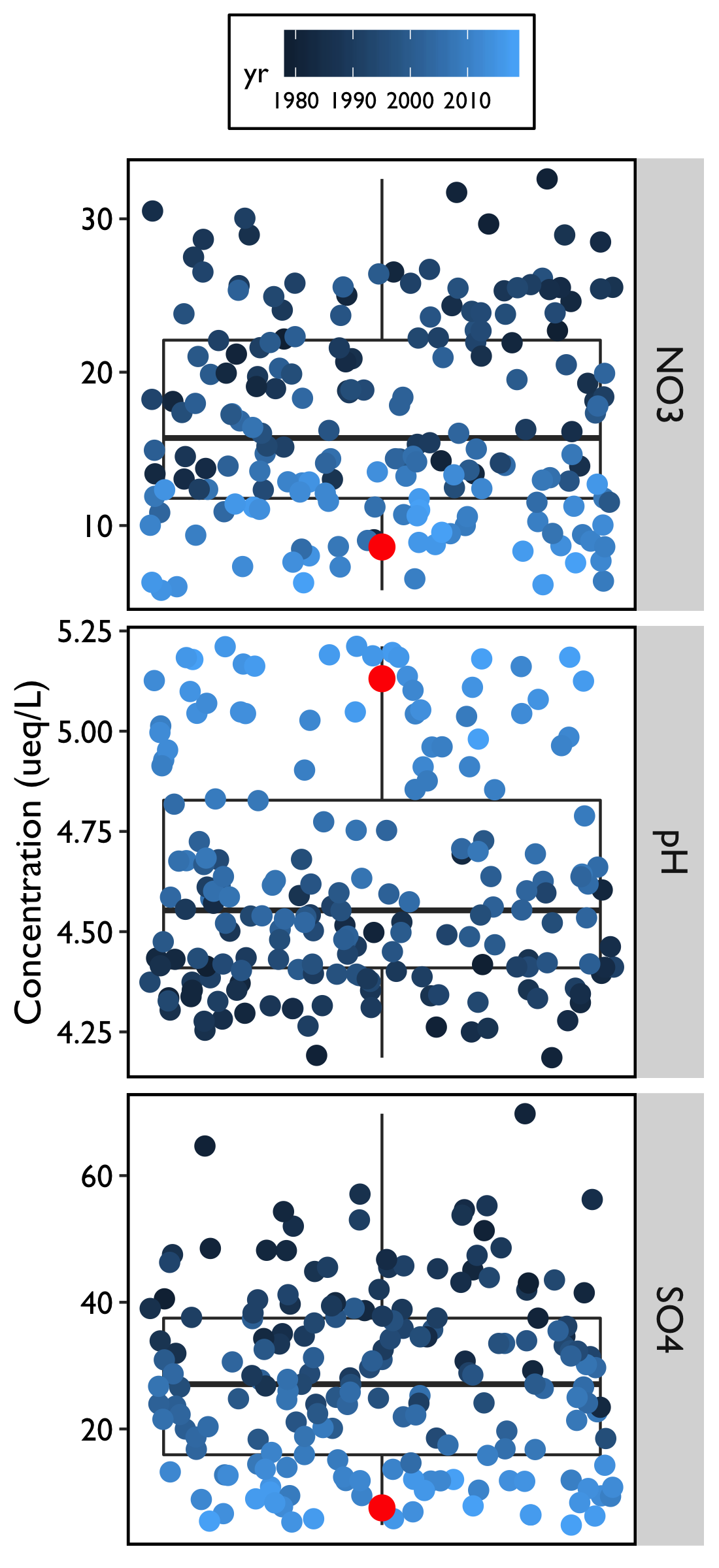
For all three metrics of acid deposition (NO3, SO4, pH), 2018 continued the trend of reduced pollution concentrations over historical measurements (Figure 11).
While mean deposition of NO3 in 2018 was not the lowest value observed in the record (Figure 11), it was the second lowest at 9.56 µeq/L, and was a considerable decline from the record high of 28.13 µeq/L in 1985. Further, for every year in the most recent decade (2006 on), precipitation contained the lowest measured concentrations of NO3.
In 2018, we saw the lowest concentration of SO4 in the record (5.45 µeq/L), continuing a five-year streak of record lows. This is a dramatic decline from the historical high of 48.20 µeq/L in 1982. For the fifth year in a row, deposition of SO4 fell below that of NO3.
The average pH was the highest on record in 2018 at 5.185, which indicates that precipitation in the form of rain, snow, or ice is continuing to be less acidic when compared to historic records. While the pH has increased considerably from the record’s low of 4.32 in 1989, “unpolluted” rain typically has a pH of 5.6. Therefore, there is still room for continued improvement in lowering the acidity of precipitation. As pH is a logarithmic scale, this increase represents a roughly fivefold improvement in precipitation acidity.
In the early years of acid rain monitoring in Vermont, SO4 accounted for about 66% of the acidity in precipitation, while NO3 contributed the other 33%. According to the U.S. EPA National Emissions Inventory 2014 Report V2, national emissions of the precursor pollutants of SO4 and NO3 have decreased substantially since 1990 levels. Sulfur dioxide (SO2) emissions have decreased by 88% since 1990, while emissions of nitrogen oxides have decreased by 58%. While the stress imposed by sulfate deposition has been greatly reduced, it is unclear how the continued deposition of nitrate will impact forested ecosystems.
Long-term Trends
Since precipitation chemistry was first measured in Vermont, rain has become less acidified (Figure 12). These changes reflect declines in sulfur- and nitrogen-based emissions due to the Clean Air Act (1977) and subsequent amendments (1990). Sulfate deposition has fallen from nearly 50 µeq/L to less than 10 µeq/L (Figure 12).
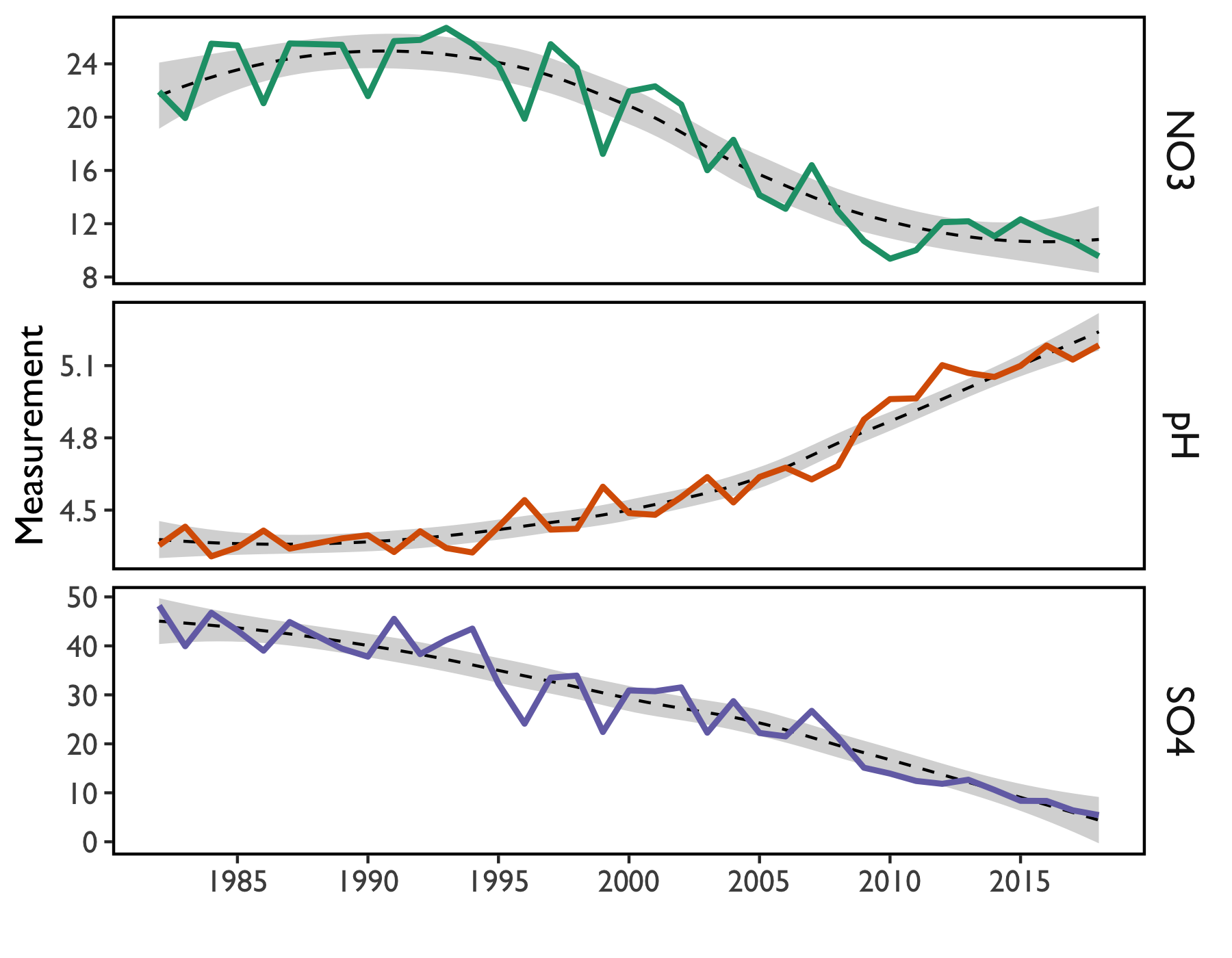
More modest changes have been measured for nitrate deposition. This is primarily due to the relative difficulty of removing nitrogen compounds from flue gases and their diffuse pollution sources such as motor vehicle exhaust and agricultural activities. Sulfuric emissions have been easier to control through regulation of emissions from the burning of coal, natural gas, and other fossil fuels, including the implementation of low sulfur fuel oil standards for heating oil.
Concurrently, there has been a dramatic increase in precipitation pH (Figure 12). Since pH is on a logarithmic scale, increasing pH by a value of 1 signifies a tenfold decrease in precipitation acidity.
Looking forward, it is likely that reductions in SO4 may continue (Figure 12), along with resultant decreases in precipitation acidity. However, it appears that reductions in NO3 concentrations may have plateaued, even though concentrations are down from last year’s measurement. Because nitrogenous pollution primarily comes from diffuse sources such as automobile exhaust, fertilizer use, and confinement farming such as feedlots and poultry operations in agricultural regions, continued reductions may require additional legislative or regulatory action.
Regional Context & Implications
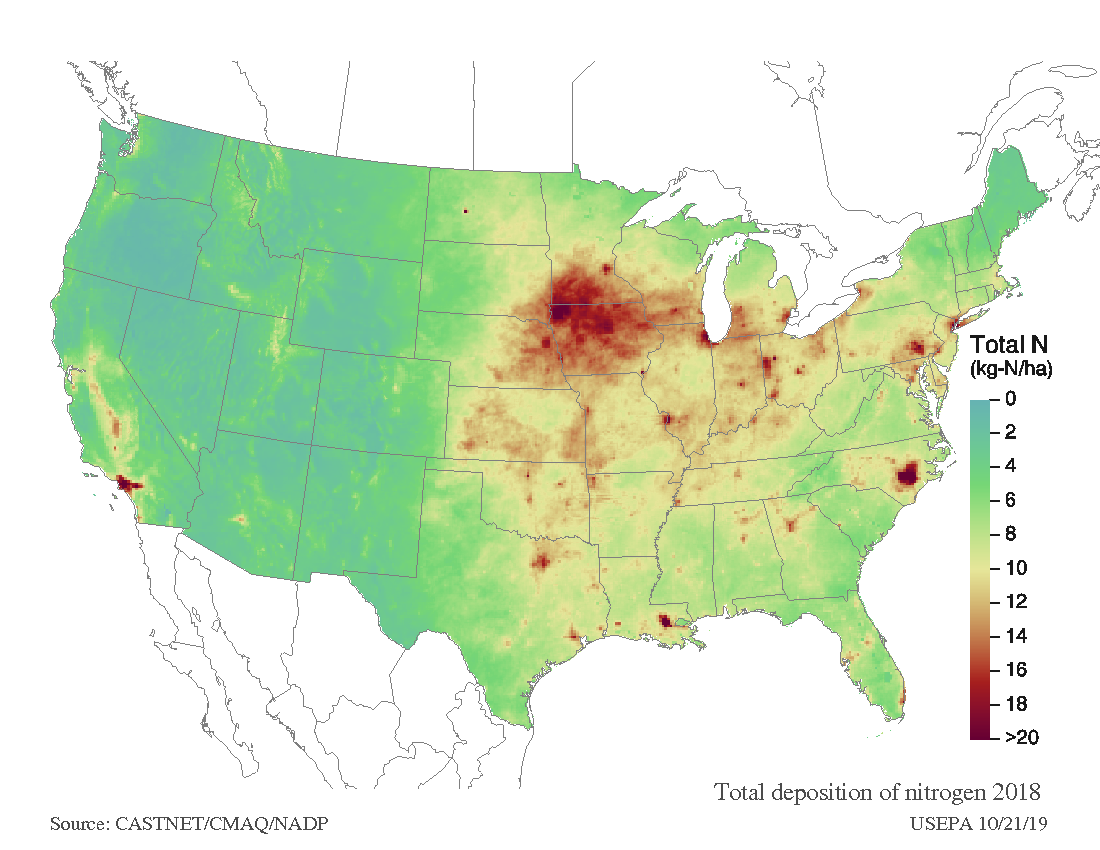
Vermont is in relatively good shape compared to nitrogen pollution loads nationwide (Figure 13). However, forests along the spine of the Green Mountains continue to be at risk from additional acidic inputs due to more frequent exposure to acid mist in clouds, higher amounts of precipitation, and relatively shallow acidic soils. As nitrogen becomes a more important constituent of acid deposition, monitoring networks and modelers are combining resources to better understand the spatial and temporal patterns of nitrogen deposition and its impacts on terrestrial and aquatic ecosystems.
Similar trends in reduced acidity of precipitation has been seen elsewhere in the region, however western and southern portions of New York continue to receive elevated deposition (Figure 13). Many areas in the Midwest US have been experiencing very high levels of nitrogen deposition; these regions are characterized by developed manufacturing industries. As a result, continued declines in nitrate deposition may require additional regulations.

• Acid deposition continued to decline in 2018.
• The average pH of precipitation was 5.185, the highest value on record.
• Nitrate deposition declined in 2018 but long-term trends suggest it may have plateaued. Continued monitoring is recommended.
Additional Resources
National Atmpospheric Deposition Program. http://nadp.slh.wisc.edu/ntn/
EARTH: The Science Behind the Headlines. American Geosciences Institute. http://www.earthmagazine.org/
2014 National Emissions Inventory Report, Version 2: https://www.epa.gov/sites/production/files/2018-06/documents/nei2014v2_tsd_09may2018.pdf
FEMC Project Database Links
National Atmospheric Deposition Program/National Trends Network (NADP/NTN) https://www.uvm.edu/femc/data/archive/project/national-atmospheric-deposition-programnational-trends-network
