What is Small Vessel Disease (SVD)?
SVD refers to pathological processes that affect the structure and function of small penetrating vessels within the brain. Among the important advances in the field of small vessel disease (SVD) has been the identification of genes involved in two Mendelian forms of non-hypertensive adult-onset SVD—CADASIL and CARASIL—that share a number of clinical and pathological features, including marked death of medial smooth muscle cells (SMCs) of small vessels in the brain.
Several lines of evidence indicate that genetic susceptibility factors contribute to the occurrence of sporadic SVD as part of a multifactorial predisposition5. Overall, CADASIL/CARASIL and the sporadic forms of SVD closely resemble one another on neuropathological and clinical levels. This suggests that monogenic (CADASIL/CARASIL) and common non-Mendelian forms of SVD may have some of the same molecular underpinnings. Indeed, there is growing evidence that variants in genes that underlie Mendelian diseases may also modulate the risk for complex forms of the same disease1.
Neurovascular Coupling (NVC)
When neuronal activity increases in a region of the brain, so does the energy demand of the active cells. However, the brain lacks intrinsic energy stores, despite using 20% of inspired oxygen and 25% of consumed glucose. So to support ongoing neuronal function, dynamic regulation of oxygen and glucose supply to match changes in metabolic demand is critical28. A continuous supply of these nutrients, matched to local demand, is made possible by a process termed ‘neurovascular coupling’ (NVC). The term neurovascular coupling encompasses the signaling cascades that enable the communication of increased neuronal activity, via support cells called astrocytes, to nearby parenchymal arterioles to evoke vasodilation and provide an increase in blood flow.
Increases in neuronal activity must be accompanied by rapid (seconds), spatially localized elevations of oxygen and glucose to avert the development of ischemic conditions29-32. This linkage between neuronal activity and local blood flow—termed functional hyperemia (or NVC)—has been appreciated for over 100 years28. However, the mechanisms by which increased synaptic activity is communicated to the cerebral microcirculation to generate a vasodilatory response are poorly understood. Recent evidence has shown that functional hyperemia is controlled by the release of vasoactive agents that act locally to dilate arterioles10,29,38,40,42,46,47,49,51. In addition to potential signaling between neurons and arterioles, accumulating evidence suggests that astrocytes, which form close associations with neurons and PAs and possess numerous receptors that may be activated by neurotransmitters36,37,45,47, play a significant role in the regulation of local CBF29,38,40,43,46,51. Activation of mGluRs located on astrocytic projections surrounding synapses of glutamatergic neurons results in an increase in cytosolic calcium in the soma. This increase propagates through astrocytic processes, ultimately resulting in a calcium increase in the endfoot that is followed by dilation of the associated arteriole35,38,44. The vasodilatory response to activation of astrocytic mGluRs is strictly dependent on increases in astrocytic endfoot calcium10,29,38,49-51; thus, the release of vasoactive substances from astrocytes requires elevation of endfoot calcium10,29,38,43,51, although the mechanisms underlying the generation of these signals and the nature of these mediators are not fully understood. Among the mediators that have been proposed to modulate vessel diameter in response to neuronal activity are carbon dioxide41 and several signals based on AA metabolism, including PGE2, EETs and 20-HETE29,34,39,43,51.
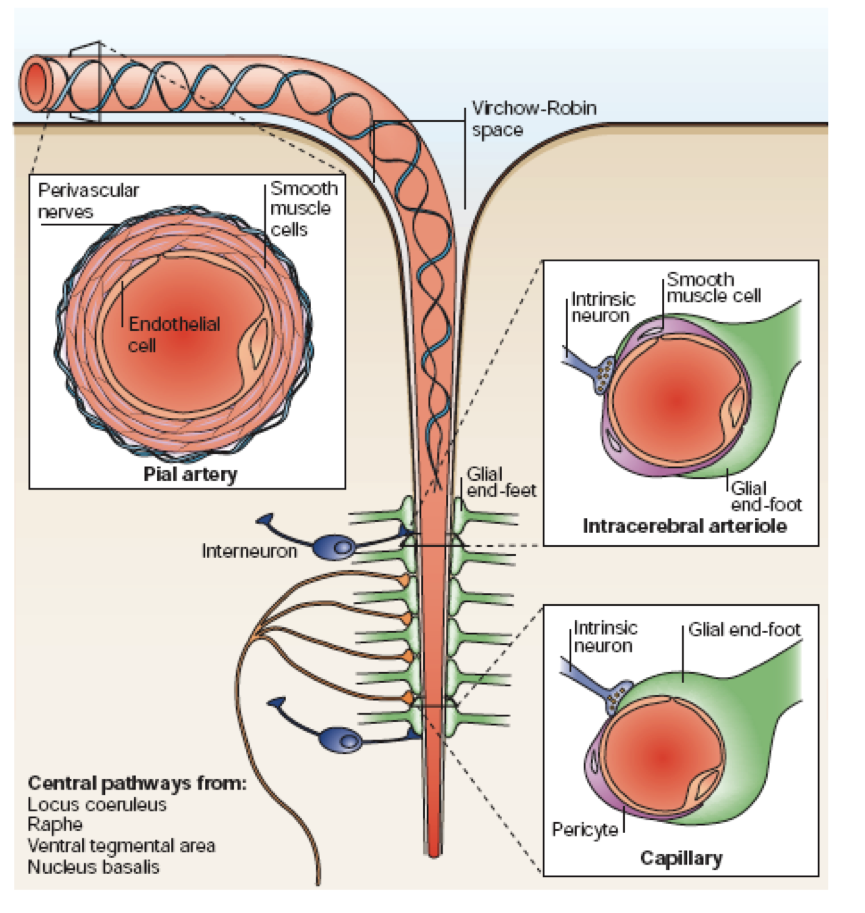
Illustration of the neurovascular unit, showing arterioles in the brain encased by astrocytic processes.
From Iadecola. Nat Rev Neurosci 2004; 5:347-360. Review.
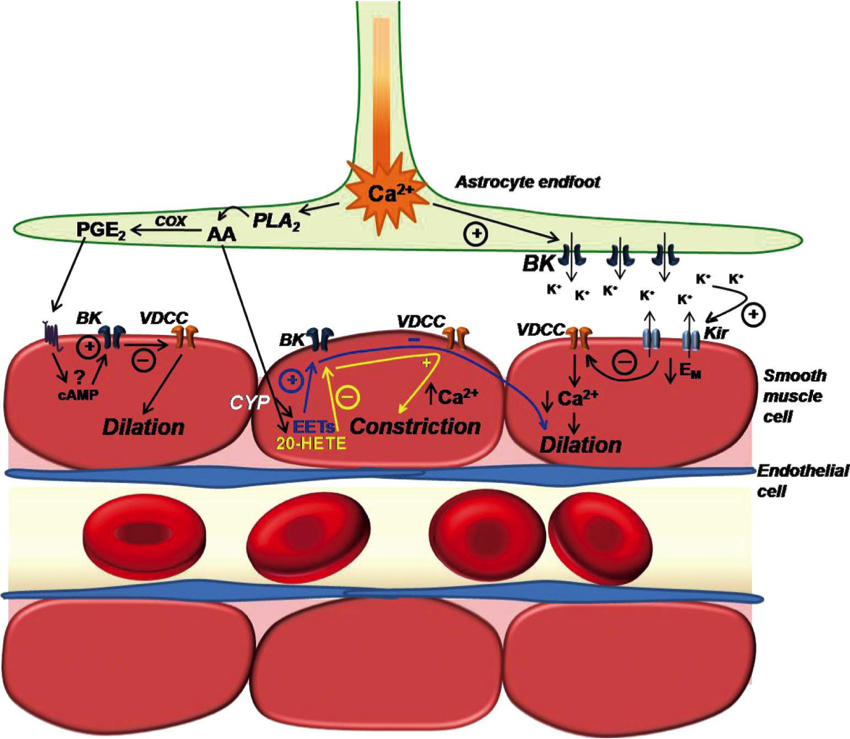
Illustration depicting the involvement of parenchymal arteriolar smooth muscle cell (SMC) K+ channels in putative mechanisms of neurovascular coupling. Neuronal activity stimulates astrocytic metabotropic glutamate receptors (not shown) to produce a propagating rise in [Ca2+]i that terminates in perivascular endfeet.
From Dunn and Nelson. Circulation Journal 2010; 74(4):608-616. Review.
K+ Channels and NVC
Dr. Mark Nelson examines the consequences of CADASIL mutations on potassium channel function in intracerebral arterioles.
Hypertension and Ageing
Dr. Frank Faraci describes the lessons learned from animal models about the effects of ageing and hypertension on cerebrovascular disease.
Franck Faraci - Hypertension and ageing... by SINGER-POLIGNAC
