What is The Leducq Center Against Small Vessel Disease ?
Understanding cerebrovascular function and dysfunction presents a daunting challenge to both clinicians and basic scientists. Until now, a research network on SVD has not been possible owing to a lack of appropriate mouse models, difficulties in adapting molecular and imaging techniques to the mouse brain microcirculation, and the absence of a critical mass of scientific information and expertise. The convergence of emerging technologies and growing insight into cerebral SVD processes—made possible in large part by the research efforts of members of the Network-create a moment in time ripe for a comprehensive and productive exploration of monogenetic (CADASIL/CARASIL) and common forms of SVD.
The Leducq Center Against Small Vessel Disease (LCASVD) encourages collaborative and interactive scientific investigation into causes of — and solutions for — Small Vessel Disease and related conditions.
Our Goal: Understanding Cerebral Blood Flow
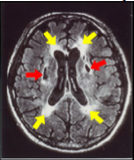
Brain MRI of a patient with SVD showing lacunar infarcts (red arrows)and white matter lesions (yellow arrows)
Small vessel disease (SVD) of the brain accounts for ~25-30% of ischemic strokes and is a leading cause of cognitive decline and disability23. SVD refers to pathological processes that affect the structure and function of small penetrating vessels within the brain. The salient consequences of SVD are small, deep infarctions in the white and/or deep gray matter, and the development of widespread white matter lesions that may or may not produce acute clinical symptoms, but do contribute to cognitive and motor impairment. SVD appears driven by a complex mix of genetic and environmental factors, among which age and arterial hypertension are currently deemed the most important13,23.
Despite its impact on the brain, there are currently no specific treatments for SVD, and therapeutic options for secondary prevention are particularly limited compared to other common causes of stroke. This is mainly due to a poor understanding of vascular disease pathogenesis9. Small vessels of the brain have unique functional properties that help ensure that the brain, which has a limited fuel reserve, receives an adequate supply of blood under a variety of conditions. In the brain, increased cellular activity is accompanied by an increase in local cerebral blood flow (CBF) that serves to satisfy enhanced glucose and oxygen demand, a phenomenon known as functional hyperemia or neurovascular coupling (NVC)17. Studies suggest that several mediators can be involved in NVC, including glutamate receptors, calcium signaling, potassium channels, nitric oxide, prostaglandin E2 and epoxyeicosatrienoic acids10,12. Cerebral arteries and arterioles also constrict or dilate when intravascular pressure increases or decreases, respectively. This phenomenon, known as myogenic reactivity, is a major contributor to the maintenance of CBF (autoregulation) during variations in systemic blood pressure16.
