![]()
Vice President for Research Resources
NIH Sample Dissemination Plan - used with Clinical Trial Submissions
Other Resources
Research Data Management Plans
Research Data Management Plans (DMP) refer to formal documentation that describes how research data is stored, accessed and preserved for future use. Planning ahead about how to manage research data ultimately saves time and ensures data is properly stored, documented, reproducible, and creates opportunities for collaboration. In addition, many federal grant applications now require research date management plans be included.
If you need assistance with developing a Research Data Management Plan, please contact the UVM Library Research Guide for Data Management.
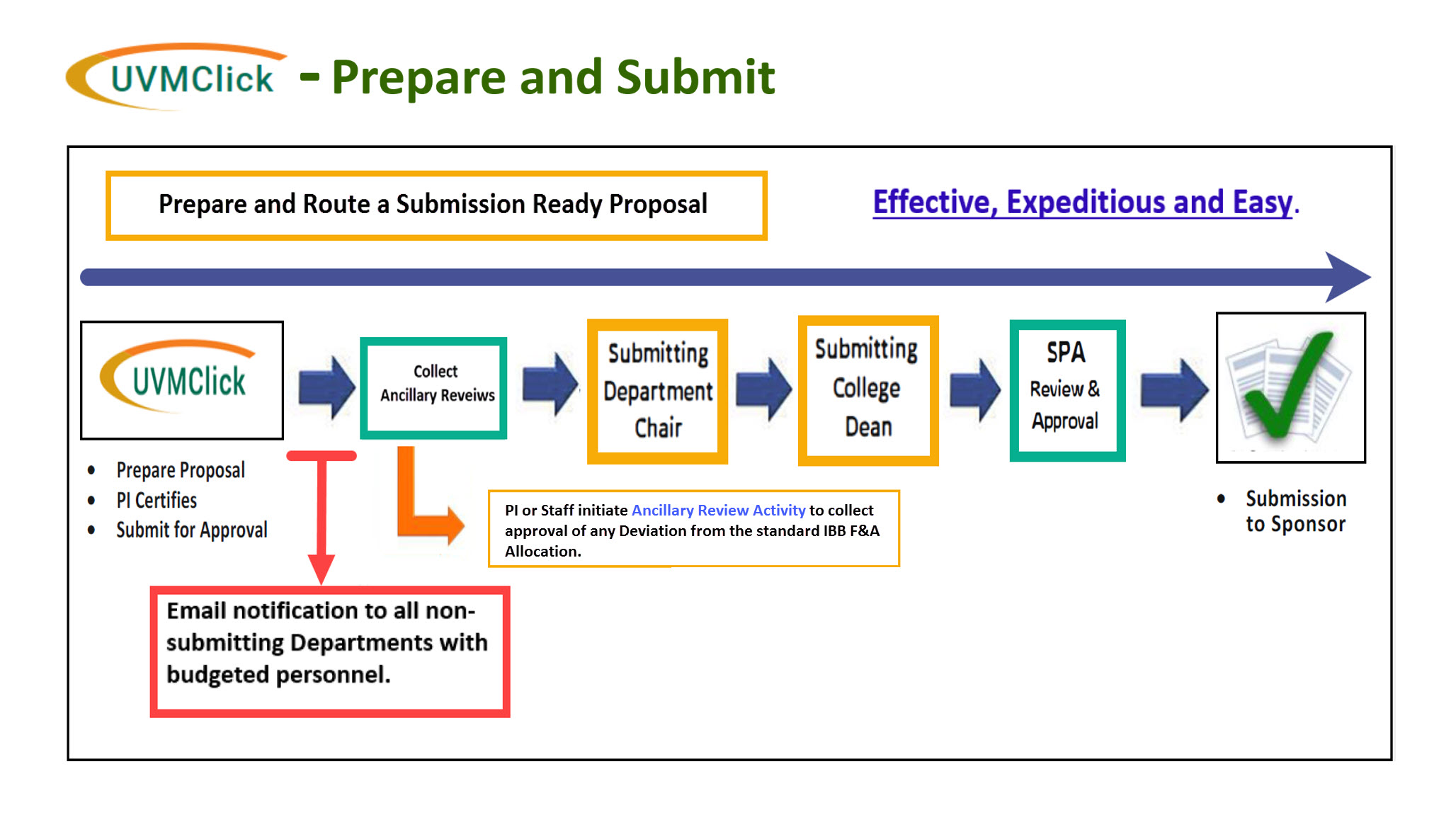
Prepare and Submit Proposals for Approvals Using UVMClick - Grants
For all new sponsored project proposals, the PI must create a Funding Proposal record in UVMClick - Grants, This includes resubmissions, competitive renewals, supplemental proposals, and pre-proposals/white papers that require detailed budgets.
Prior to submission to the sponsor, the PI submits their Funding Proposal (FP) to SPA by routing for all required institutional approvals through UVMClick. A typical proposal requires 4 levels of approval in the following sequence:
- Principal Investigator (certification)
- Department (Chair or designee)
- College (Dean or designee)
- Sponsored Project Administration (Authorized Official)
Updated 2/3/21