About TSP
Who We Are
Our team manages the overarching planning, management, and day-to-day operations of TSP at sites in Vermont, New York, New Hampshire, and Maine. Our dedication and strong work ethic have garnered awards from AAMI, 24x7 Magazine, Biomedical Instrumentation and Technology, American College of Clinical Engineering, and the Vermont Council for Quality.
To deliver the best in research enterprise and health care technology management services, we invest in the most qualified Biomedical Equipment Technicians (BMETs) and Clinical Engineers. Both play key roles in the effective management of your complete Health Care Technology Life Cycle.
What We Do
For over 50 years, Technical Services Partnership (TSP) has been partnering with university researchers and health care providers to increase the quality of research and patient care through the use of advanced medical technology. Our Technology Life Cycle planning and management services enable our customers to stay abreast of the latest advances in technology while ensuring safety, clinical efficacy, cost containment, and operational excellence. TSP's broad focus on the full spectrum of technology is unique among similar service providers. Effective Technology Management Technology plays a key role in achieving positive outcomes for researchers and for patients. However, it is a major cost driver, not only due to the initial equipment acquisition cost, but also the full life-cycle costs associated with installation, training, maintenance, consumables, compliance, and so on.
Technology Life Cycle
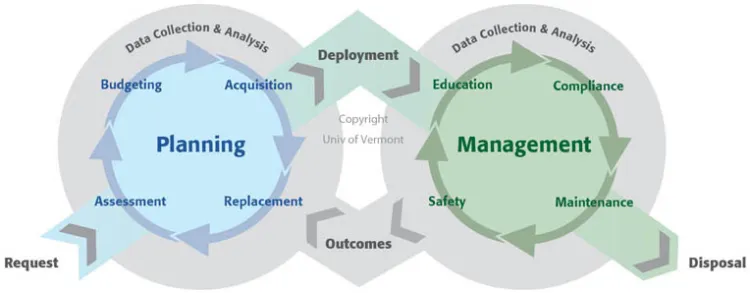
The life cycle of clinical technology begins at the thought of purchase and continues through to the disposal of the asset. The planning phase for clinical technology takes into account the key characteristics of the asset, pricing, and functionality. Once the device is deployed, the equipment enters the management phase of the lifecycle, which includes compliance testing, safety evaluations, and any incident investigations. Throughout the life cycle, continuous documentation is maintained.
Effective Technology Management
Technology plays a key role in achieving positive outcomes for researchers and for patients.
However, it is a major cost driver, not only due to the initial equipment acquisition cost, but also the full life-cycle costs associated with installation, training, maintenance, consumables, compliance, and so on.
Acquisition costs are only the tip of the iceberg.
Due to the complexities of the research enterprise and of health care systems, inefficiencies can develop. Departmental and individual efforts to implement technology can backfire if the overall health care system environment is not taken into consideration.
Lack of standardization, improper application, limited infrastructure, failing to capture economies of scale, not anticipating overall utilization, and ineffective maintenance approaches can all lead to escalating costs and less-than-optimal patient care.
For technology to be successful in enhancing research and in improving patient outcomes, a comprehensive system of technology planning and management is required: one that optimizes the capacity to produce the desired results while effectively managing costs throughout the full technology life cycle.
Acquisition Costs ~ 20%
- Technology purchase price
- Shipping
- Installation
Life Cycle Costs ~ 80%
- Staffing and training
- Maintenance
- Consumables and utilities
- Upgrades
- Software licenses
- Financing
- Compliance
- Quality assurance
- De-installation and disposal
Leadership
Administration
The ITS Administration and Leadership are dedicated to the delivery of the highest quality support services for research, healthcare and the community. Our teams are ready to help meet the needs of our clients in a timely and cost effective manner.
| Photo, Name, and Title | Role, Description, and Email |
|---|---|

Michael Lane, Director | Michael Lane, Director of ITS, earned a Bachelors in Electrical Engineering and an MBA from the University of Vermont. Mike has served ITS in multiple roles including Associate Director and Clinical Engineer and holds CHTM certification from AAMI and CMQ/OE certification from ASQ. |

Laurence Robert, Associate Director | Laurence Robert, Associate Director of ITS, earned a Bachelors in Electrical Engineering from the University of Vermont and completed an internship at ITS. Larry contributed to the HTM field by developing test equipment, leading a biomedical technician society and working to bridge the CE/IT gap. He holds certifications as a CBET and CHTM from AAMI. |

Adam Sbardellati, Business Manager | Adam Sbardellati is the Business Operations Manager with over a decade of experience in operations management. Adam brings an enthusiasm for team building and thrives on fostering collaboration and unity to achieve shared goals. |
Leadership
Our Leadership works in partnership with your staff and our Biomedical Equipment Technicians and Clinical Engineers to ensure your medical equipment is available and operating at peak performance. They are actively engaged on a day-to-day basis to maintain, troubleshoot, and repair your advanced medical devices. Many of our staff are certified by the International Certification Commission.
| Photo, Name, and Title | Role, Description, and Email |
|---|---|

Tim Agan, BMET Services Supervisor | Tim Agan is the Biomedical Supervisor for TSP’s Specialty Team. A USAF veteran, he has 38 years of wide-ranging technical experience, with 30 of these being devoted to biomedical technology at TSP. |

Richard Barnes, BMET Services Supervisor | Direct responsibility for all biomedical equipment services in North West Regional hospitals |
UVMMC, BMET Services Supervisor | Laurence Robert, Associate Director of ITS, is covering this position until is it filled, as the previous supervisor retired. |

Raymond Forsell, Clinical Engineering Manager | Raymond Forsell is a Certified Clinical Engineer, Certified Healthcare Facility Manager and Vermont Professional Engineer. He is the Clinical Engineering Manager with 41 years of experience in the profession. |

Mark Robinson, BMET Services Supervisor | Mark Robinson is a Biomedical Supervisor with over 20 years of experience in the field. A military retiree, he brings dedication and discipline to his work in biomedical technology. |
Clinical Engineers
Clinical Engineers focus primarily on the planning phases of the life cycle in areas such as technology assessment, budgeting, equipment acquisition, patient safety, education, regulatory compliance and risk analysis. They work collaboratively with your staff and our Biomedical Equipment Technicians to ensure that the most appropriate technology is acquired and effectively managed for maximum availability, and revenue production.

Raymond Forsell, Clinical Engineering Manager
- Provides clinical engineering consultation services to all New Hampshire and Vermont hospitals except University of Vermont Medical Center
- Raymond.Forsell@uvm.edu
David Fournier, Clinical Engineer
- Provides clinical engineering consultation services
- David.Fournier@uvm.edu
Zachary Munson, Clinical Engineer
- Provides clinical engineering consultation services to University of Vermont Medical Center and Vermont
- Zachary.Munson@uvm.edu
Leah Francouer, Clinical Engineer
- Primary liaison with University of Vermont Medical Center regarding TSP activities
- Provides clinical engineering consultation services to University of Vermont Medical Center
- Leah.Francoeur@uvm.edu
Haley Kuralt, Clinical Engineer
- Provides clinical engineering consultation services to New Hampshire, New York, and Maine
- Haley.Kuralt@uvm.edu
Why TSP?
Full life-cycle approach
- Full life-cycle approach to technology management results in better planning, reduced costs, and a higher standard of patient care
Custom education and advisory services
- Custom education and advisory services offered by our knowledgeable Clinical Engineers
Highly responsive
- Highly responsive Biomedical Equipment Technicians
Recognition locally, nationally and internationally
- Recognition locally, nationally and internationally for our leadership in the field of health care technology management
Ability to leverage knowledge and resources
- Ability to leverage knowledge and resources of The University of Vermont and its highly regarded biomedical engineering program
TSP History
TSP History
In the early 1970s, the Instrumentation and Model Facility (IMF) at the University of Vermont (UVM) was receiving an increasing number of requests to provide support services for the new, complex instrumentation at the Mary Fletcher Hospital. Concurrently, the Northern New England Regional Medical Program (RMP) at UVM — a regional collaboration between regional medical schools, research institutions, and hospitals — contacted IMF to request the performance of an electrical safety survey of Vermont hospitals.
It became apparent to RMP and IMF that there was an opportunity for UVM to provide cost-effective, shared clinical engineering services to the hospitals of Vermont.
Creation of Technical Services Program
In 1973, RMP agreed to support a UVM graduate student in conducting a needs assessment of all hospitals in the state of Vermont. The results of his study found that 17% of medical equipment had excessive electrical current leakage and 5% of the equipment was inoperable. A report summarizing his findings resulted in the formation of an ongoing UVM program dubbed the "Technical Services Program (TSP)." The Vermont Hospital Association agreed to coordinate an ongoing program of equipment surveys among Vermont hospitals.
Concurrently, the Universities of Maine and New Hampshire had been considering similar programs. They teamed up with UVM's TSP to submit a grant proposal to the W. K. Kellogg Foundation. This proposal was accepted by the Kellogg Foundation and the result was the formation of the Northern New England Clinical Engineering Program in November 1973. Kellogg Foundation grant funds were proportioned among the programs in Vermont, Maine, and New Hampshire and funded the early development of these organizations. UVM TSP’s initial staffing included one full-time engineer, two part-time engineers, a graduate engineering student, and a secretary.
The original mission of shared clinical engineering services as defined at a meeting of all Kellogg Foundation–funded organizations in 1973 was:
To provide health care organizations of all types and sizes with convenient and economical access to those technical support and advisory services which are now a necessary part of high-quality patient care.
Transition from Kellogg Grant
Funding from the Kellogg Foundation grant to UVM TSP continued until October 1976, at which point individual hospitals assumed responsibility for services rendered. At this time, the Vermont Hospital Association acted as a third party to all contracts and processed all invoices and payments between organizations. The TSP User's Committee was formed to monitor services and fees, and to serve as a vehicle for input into continued program development and evolution.
By this time, two hospitals in northern New York state had been added to the TSP membership. The focus of services was equipment safety and control and direct services such as purchase consultation, incoming inspection, functional testing, preventative maintenance and repair, and education. National Counsel on Radiation Protection (NCRP) compliance testing services were also conducted by TSP for all diagnostic X-ray equipment in the state of Vermont. In 1976, the first seminar co-sponsored by TSP and the Vermont Hospital Engineering Society was held. Additional northern New York hospitals were added as TSP clients in the late 1970s, a move fully endorsed by The Vermont Hospital Association. TSP services were expanded to health care facilities in northern New Hampshire in the 1980s.
Renaming of TSP to "Partnership"
In the spirit of its ongoing partnerships with hospitals and independent clinical care facilities in northern New England, TSP formally changed the name of the organization to "Technical Services Partnership" in 2010.
TSP remains a non-profit division within the University of Vermont and continues to build upon its strong heritage of providing trusted, knowledgeable and responsive service to regional health care organizations.