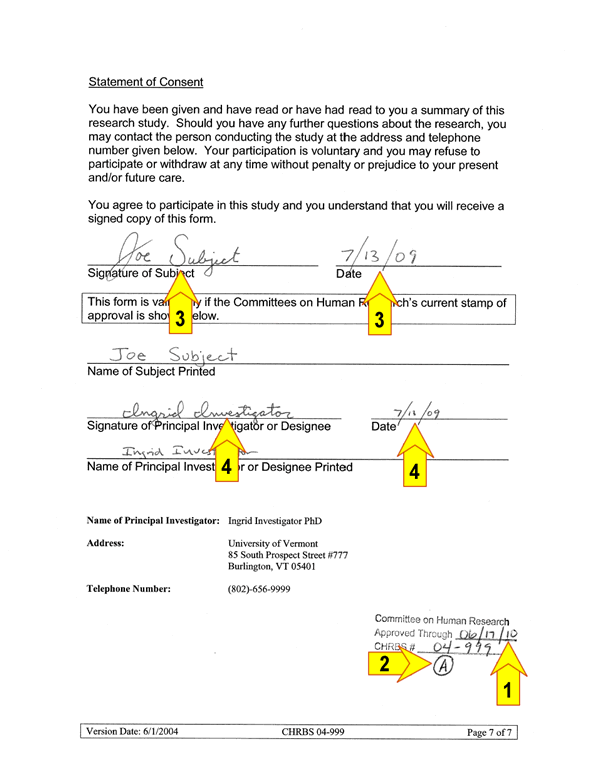
Consent Completion
When consenting participants, there are four key elements in the consent document that must be verified and completed before research activities may begin (referred to as a fully executed consent):
1 ) the expiration date on the consent document
has not passed;
2 ) the version of the consent is the most current with regards
to protocol amendments requiring consent form revisions.
3 ) the participant has signed and dated the consent;
4 ) the investigator (or designee) has signed and dated the
consent.
A completed signature page should look like this (key elements are numbered):
