Amendments
Whenever there is an amendment to a protocol which requires a change to the consent document, the IRB must approve the changes to the consent document. The IRB will stamp the new version of the consent document and return it to the researcher.
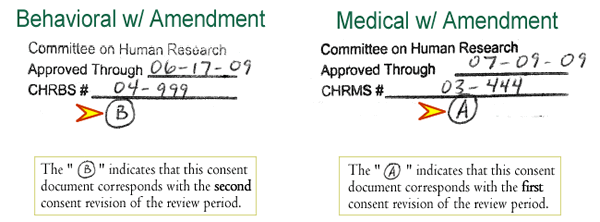
The revised consent form will be given the same expiration date (if not outdated at the time of amendment request) as the pre-amendment consent form. There will now, however, be an identifying letter (i.e., A, B, C, …) directly below the stamp. This letter is associated with the approved informed consent revision. If there are multiple informed consent revisions during the course of the year, the identifying letters will proceed alphabetically. Only the most recently approved version of the consent document can be used to consent participants, so care must be taken to verify that the most current version is being used. A procedure should be in place to notify all consenting researchers that there has been an amendment to the protocol that requires the use of the newly revised and IRB approved consent form. At continuing review, a new expiration date will be given and the letters will begin again under that new expiration date.
The IRB stamp, with the identifying letter, will look like this:
