1. DEFINITIONS
AND REGULATORY AGENCIES
BioHazardous Materials
For
purposes of the IBC Policies, biohazardous materials include, but are not
limited to, the materials defined in this section.
Recombinant
or Synthetic Nucleic Acid Molecules
The NIH Guidelines for
Research Involving Recombinant or Synthetic Nucleic Acid Molecules defines these as (i) molecules that a) are constructed by
joining nucleic acid molecules and b) can replicate in a living cell (i.e.
recombinant nucleic acids);
(ii) nucleic acid molecules that are chemically
or by other means synthesized or amplified, including those that are chemically
or otherwise modified but can base pair with naturally occurring nucleic acid
molecules (i.e. synthetic nucleic acids); or
(iii) molecules that result from the
replication of those described in (i) or (ii) above.
https://osp.od.nih.gov/biotechnology/nih-guidelines/ Synthetic nucleic acid segments that are likely to yield a potentially harmful polynucleotide or polypeptide (e.g., a toxin or a pharmacologically active agent) are considered as equivalent to their natural nucleic acid counterpart.
Infectious
Biological Agents
Infectious biological agents include biological
agents and biologically derived materials that present a risk or potential risk
to the health of humans or animals, either directly through infection or
indirectly through damage to the environment.
Categories of potentially infectious biological
materials include the following:
·
Human,
animal, and plant pathogens (bacteria, parasites, fungi, viruses, prions).
·
All
human blood, blood products, tissues, and certain body fluids when used in
conjunction with infectious agents or recombinant or synthetic nucleic acid molecules.
·
Cultured
cells and potentially infectious agents these cells may contain.
·
Clinical
specimens.
·
Infected
animal and animal tissues.
Biotoxins
A biotoxin is a poisonous substance that is a
specific product of the metabolic activities of a living organism and is
usually very unstable, notably toxic when introduced into the tissues, and
typically capable of inducing antibody formation. Biological toxins can include metabolites of
living organisms, degradation products of dead organisms, and materials
rendered toxic by the metabolic activity of microorganisms. Some toxins can also be produced by bacterial
or fungal fermentation, by the use of recombinant or synthetic nucleic acid
moleculestechnology, or by chemical syntheses of low molecular weight toxins. Biological toxins behave like chemical toxins
in that they are non-replicating and therefore are not considered
infectious. Since they exert their
adverse health effects through intoxication, the toxic effect is analogous to
chemical poisoning rather than to a traditional biological infection.
Select
Agents and Toxins
Select agents and toxins
are those biological agents and toxins that are deemed to pose a threat to
public, animal or plant health. The
Department of Health and Human Services (HHS), Center for Disease Control and
Prevention (CDC), and the United States Departmet of Agriculture (USDA) have
identified those select agents and select agent toxins (“listed select agent or
toxin”) that are subject to protocol and regulatory oversight. The HHS/CDC lists of select agents and toxins
(include those that overlap with the USDA) are identified at 42 CFR 73.3 (HHS
list) and 42 CFR 73.4 (Overlap list).
The USDA list of select agents and toxins are identified at 9 CFR
121.3. The CDC list of all select agents
and toxins is located at
https://www.selectagents.gov/selectagentsandtoxinslist.html This list is updated on an ongoing basis at http://www.selectagents.gov/.
Regulatory Agencies
Office of
Biotechnology Activities (OBA)
Monitors scientific progress in human genetics
research in order to anticipate future developments, including ethical, legal,
and social concerns, in basic and clinical research involving Recombinant DNA,
Genetic Technologies, and Xenotransplantation;
Recombinant
DNA Advisory Committee (RAC)
The
Recombinant DNA Advisory Committee (RAC) was established by the NIH on October
7, 1974 in response to public concerns regarding the safety of manipulating
genetic material through the use of recombinant DNA techniques.
The
RAC developed a set of guidelines, now known as the NIH Guidelines for
Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH
Guidelines). While compliance with the NIH Guidelines is
mandatory for investigators at institutions receiving NIH funds for research
involving recombinant DNA, they have become a universal standard for safe
scientific practice in this area of research and are followed voluntarily by
many companies and other institutions not otherwise subject to their
requirements.
In
addition to seeking the RAC’s advice on needed changes to the NIH Guidelines,
the NIH asks the RAC to consider other matters pertinent to basic and clinical
research involving recombinant DNA. A major responsibility of the RAC at
present is to review human gene transfer research on behalf of the NIH.
Human gene transfer trials conducted at, or sponsored by, institutions
receiving NIH funding for recombinant DNA research are registered with OBA and
reviewed by the RAC.
2. COMMITTEE MISSION
The
University of Vermont (UVM) is committed to minimizing the risks to faculty,
staff, students, the public, the facilities, and the environment while using biohazardous
materials during research at UVM. The
Institutional Biosafety Committee (IBC) is responsible for ensuring the biohazardous
materials as defined above are used in research safely and appropriately. IBC policies for review and use of these biohazardous
materials apply to research that is:
•
Sponsored by UVM,
•
Conducted by UVM personnel, or
•
Conducted using UVM’s property, facilities, or non-public information.
The IBC Policies are based upon the following regulations and
guidelines:
NIH Guidelines for Research Involving Recombinant
or Synthetic Nucleic Acid Molecules (NIH Guidelines)—
This document provides guidelines for constructing and handling recombinant
and synthetic nucleic acid molecules and organisms containing recombinant or
synthetic nucleic molecules. This document requires that each institution
establish an Institutional Biosafety Committee with the authority to approve
proposed recombinant and synthetic nucleic acid molecule research using the NIH
Guidelines as a minimum standard. The NIH Guidelines publication is available
at https://osp.od.nih.gov/biotechnology/nih-guidelines/
Biosafety in Microbiological and Biomedical Laboratories (BMBL),
published
by Centers for Disease Control and Prevention (CDC) and NIH - This
document contains guidelines for microbiological practices, safety equipment,
and facilities that constitute the four established biosafety levels. The BMBL
is generally considered the standard for biosafety. The BMBL is available at
http://www.cdc.gov/biosafety/publications/bmbl5/index.htm
3. COMMITTEE RESPONSIBILITIES/AUTHORITY
The
IBC is responsible for establishing and implementing policies that (1) provide
for the safe use of certain biohazardous
materials in research, and (2) ensure compliance with appropriate
federal requirements, including the NIH Guidelines and the BMBL. The
responsibilities of the IBC include, but are not limited to, the following:
·
Define the basic policies, procedures and standards as
required by NIH to oversee the safe use of these biohazardous materials (also
referred to hereafter as “these materials”.)
·
Review
requests for the use of these materials for
compliance with NIH Guidelines and the BMBL, and approve those requests which
are found to conform with NIH Guidelines and the BMBL. As part of the review
process, the IBC will do the following, as applicable:
o
Conduct
a a risk assessment to determine contaminent level, as required by the NIH
Guidelines for research involving recombinant and synthetic nucleic acid
molecules.
o
Conduct
an assessment, if applicable, of the facilities, procedures, practices,
training, and expertise of personnel
involved in the requested use of these materials.
o
Ensure
compliance with all surveillance, data reporting, and adverse event reporting requirements
set forth in the NIH Guidelines.
·
Disapprove, terminate, or suspend activities involving
these materials which are not in conformity with the Guidelines;
·
Notify investigators in writing of its decision to
approve or withhold approval of activities involving these materials, or of
modifications required to secure IBC approval.
All decisions will be part of the IBC records maintained by the Research
Protections Office;
·
Set containment levels as specified in the NIH
Guidelines and BMBL;
·
Conduct periodic review of the use of these materials to
ensure that the requirements of the Guidelines are being fulfilled;
·
Assist
the University’s Office of Risk Management in maintaining and following
emergency plans covering accidental spill and personnel contamination resulting
from use of biohazardous materials;
·
Report to the Vice President for Research any
significant related illness or accident resulting from use of these materials
that appears to be a hazard to public health;
·
Report to the Vice President for Research and the NIH
Office of Biolotechnology Activities (OBA) any significant problems with or
violation of the Guidelines;
·
The
IBC may not authorize initiation of experiments with the materials within its
purvue not explicitly covered by the Guidelines until NIH (with the advice of the
RAC when required) establishes the containment requirement.
·
Perform
post approval monitoring
4. COMMITTEE
CONTACTS
https://www.uvm.edu/rpo/contact-us
The administrative office of the IBC is located
in 213 Waterman Bldg, 85 South Prospect St,
Burlington, VT 05405, (802) 656-5040.
The RPO staff as well as a list of the current Committee Chairs and the
Veterinarian is located under contacts on our website.
5. TYPES OF COMMITTEE REVIEW
Regardless
of type of review, researchers must submit completed protocol forms for review
if engaging in research with the materials defined above.
5.A. Full Prior Review of Non-Exempt Research
For
projects requiring full IBC review (non-exempt biohazardous materials in a risk
category greater than or equal to Biosafety Level 2 (BSL2), the researcher must
complete a Protocol Form and submit to the IBC Committee for review. The IBC may take one or more of the following
actions:
•
Approve the project without modification.
•
Approve the project subject to stipulations and/or minor modifications.
•
Table the decision pending additional information.
•
Disapprove the project.
The
researcher may not initiate the project until IBC approval is given.
5.B.
Review Simultaneous with Initiation
Those projects determined to have a risk category BSL1 may be initiated
prior to approval. The determination that
the research fits within the “review simultaneous” category will be made by the
Biological Safety Officer and the Chair.
The researcher will receive a memo stating that activity may begin. The project will be placed on the next
available agenda for full IBC review. The
researcher may be asked for further clarifications after the full review and the IBC may take one or
more of the following actions:
•
Approve the project without modification.
•
Approve the project subject to stipulations and/or minor modifications.
•
Table the decision pending additional information.
•
Disapprove the project.
5.C. Review of Exempt Research
According
to federal regulations, IBC review is not required for certain categories of
research activities that involve little or no risk to research personnel. However, the University has an obligation to
be apprised of all potentially biohazardous materials being used under its auspices
in the event any questions or problems arise and in order to assure that,
regardless of risk, all University personnel as well as the environment are protected. Therefore, these projects must be registered
with the IBC by the submission of protocols.
Exempt
status, as defined by the NIH Guidelines, will be determined by the Biological
Safety Officer and confirmed by the Chair, or designee. The
Chair or designee may request a review by the full IBC if there is question
regarding the project’s status. Exempt
protocols will not be subject to further IBC review.
Note that any revisions to the research
affecting the biosafety level may affect the determination of exemption and
therefore must be prospectively submitted for review to confirm the status.
5.D. Designated Review (new)
Projects deemed Equivalent
to a Previously Approved Project
The IBC may determine that a
proposed project is equivalent to a project that has previously been
approved. A project will only be
considered equivalent if, as determined by the IBC, there are no substantive
differences that would change the biosafety and or public health considerations
for the proposed project.
The initial determination is made by the Biosafety Officer and
Chair. The project will then be sent out
for designated review to the full Committee for a period of 5 days. If no additional clarifications or requests
for a Full Committee review at a convened meeting are received, an approval
will be signed at the discretion of the chair.
5.E. Review of Dual Use Research of Concern (New
Section)
Dual use research of
concern (DURC) is life sciences research that, based on current understanding,
can be reasonably anticipated to provide knowledge, information, products, or
technologies that could be directly misapplied to pose a significant threat to
public health and safety, agricultural crops and other plants, animals, the
environment, material or national security.
On March 29, 2012, the
U.S. Government (USG) issued its “Policy for Oversight of
Life Sciences Dual Use Research of Concern” (March 29 Policy). The policy formalizes a requirement of
regular Federal review of USG-funded or -conducted research with certain
high-consequence pathogens and toxins. Funders
and recipients of life sciences research have a shared responsibility for
oversight of DURC. The oversight applies to all DURC-related projects,
regardless of the source of funding.
Scope of Oversight Required
Under this Policy
Consistent with the March 29 USG Policy, life sciences research
that uses one or more of
the agents or toxins listed
below, and produces,
aims to produce, or can be
reasonably anticipated to produce one or more of the experimental effects listed
below, must be evaluated
for DURC potential.
|
Avian influenza virus (highly
pathogenic) |
Marburg virus |
|
Bacillus anthracis |
Reconstructed 1918 Influenza virus |
|
Botulinum neurotoxin |
Rinderpest virus |
|
Burkholderia
mallei |
Toxin-producing
strains of
Clostridium botulinum |
|
Burkholderia
pseudomallei |
Variola major virus |
|
Ebola virus |
Variola minor
virus |
|
Foot-and-mouth
disease virus |
Yersinia
pestis |
|
Francisella
tularensis |
|
Categories of experimental
effects
a) Enhances the harmful consequences of the agent or toxin
b) Disrupts immunity or the effectiveness of an immunization against the agent or toxin without clinical and/or agricultural justification
c) Confers to the agent or toxin resistance to clinically and/or agriculturally useful prophylactic or therapeutic interventions against that agent or toxin or facilitates their ability to evade detection methodologies
d) Increases the stability, transmissibility, or the ability to disseminate the agent or toxin
e) Alters the host range or tropism of the agent or toxin
f) Enhances the susceptibility of a host population to the agent or toxin
g) Generates or reconstitutes an eradicated or extinct agent or toxin listed above.
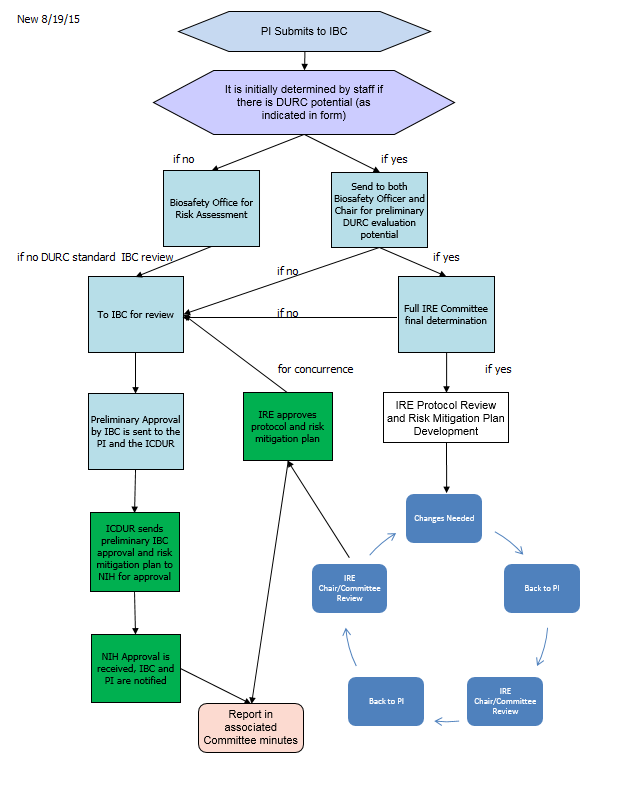
Mechanism for
PIs to Submit Potential DURC
The IBC modified its protocol submission form to gather
information needed to determine if the project may include DURC. Based on the information submitted in the
form, if the potential for DURC exists, the project is referred to the Chair of
the IBC and the Biosafety Officer, and other specialists as needed, for an
initial evaluation of potential of DURC. If the potential exists, the project
is then referred to the Institutional Review Entity (IRE) for further review. If the potential does not exist, the project will
undergo IBC review.
The IRE is tasked with initial concurrence of DURC and
then works with the PI on development of a risk mitigation plan. The following flow demontrates the review and
approval flow of a DURC project.
6. RISK ASSESSMENT AND SUBMISSION OF INITIAL AND SUBSEQUENT PAPERWORK
6.A. Initial
Risk Assessment
Research involving these biohazardous
materials is classified on the basis of perceived risk to humans and the
evironment. The risk classification determines the type of biological and
physical containment level. It is the
responsibility of the researcher to meet with the Biological Safety Officer to
conduct a risk assessment to determine the appropriate level of perceived risk
and biological and physical containment level prior to using these biohazardous
material(s). The risk assessment and the
Biosafety Officer’s signature are required to be on the protocol form prior to
submission for review. Therefore, you should plan to
complete this visit at least four weeks prior to an IBC Committee meeting
date. See meeting schedules on the Committee
forms page.
6.A.1. Risk Groups
|
Risk Groups |
|
|
Risk Group 1 (RG1) |
Agents that are not associated with disease in healthy adult humans. (BSL-1) |
|
Risk Group 2 (RG2) |
Agents that are associated with human disease which is rarely serious and for which preventive or therapeutic interventions are often available. (BSL-2) |
|
Risk Group 3 (RG3) |
Agents that are associated with serious or lethal human disease for which preventive or therapeutic interventions may be available (high individual risk but low community risk). (BSL-3) |
|
Risk Group 4 (RG4) |
Agents that are likely to cause serious or lethal human disease for which preventive or therapeutic interventions are not usually available (high individual risk and high community risk). (BSL-4) |
The
following factors will be considered when conducting a risk assessment and
determining the level of containment:
•
Pathogenicity of the biohazardous material(s) - Consideration should
include disease incidence and severity.
•
Route of transmission (e.g., parenteral, airborne, by ingestion) - When
planning to work with a relatively uncharacterized agent with an uncertain mode
of transmission, the potential for aerosol transmission should be strongly
considered.
•
Agent stability - Should include a consideration of factors such as
desiccation, exposure to sunlight or ultraviolet light, or exposure to chemical
disinfectants.
•
Infectious dose of the agent and communicability - Consideration should include the range from the healthiest
immunized worker to the worker with lesser resistance.
•
Concentration - Include consideration of the milieu containing the
organism (e.g., solid tissue, viscous blood or sputum, liquid medium) and the
activity planned.
•
Volume - >10 liters is considered
large scale and is subject to further review and higher containment level.
•
Origin of the biohazardous material(s) - Consideration should include factors such as geographic
location, host, and nature of the source.
•
Availability of data from animal studies - This information may be
useful in the risk assessment process in the absence of human data.
•
Established availability of immunization/vaccine or treatment - The unavailability of
immunization/vaccine or treatment may impact the risk involved in the use of
biohazardous material(s).
•
Gene product effects, such as toxicity, physiological activity, and
allergenicity.
6.A.2. Biosafety Level (Biological And Physical Containment Level)
The
final risk assessment determination is used to set the appropriate biosafety
level (BSL-1 to BSL-4) for the biohazardous material(s). The biosafety level
describes the degree of physical containment and biosafety practices required
to confine these materials and to reduce the potential for exposure of
laboratory workers, persons outside the laboratory, and the environment. Containment and biosafety practice are the
same unless otherwise designated. UVM
does not have any laboratories certified for BSL-4, therefore no use or
possession of biohazardous materials requiring BSL-4 is allowed at UVM.
The IBC will make the final decision as to appropriate biological
and physical containment levels for the biohazardous materials subject to its
review and approval.
6.B. Initial
Submission of Protocol
Once the Biosafety Officer’s risk
assessment and signature have been obtained on the protocol, the researcher submits
one signed original of the Protocol Form, to the IBC by the deadline which is at
least two weeks prior to the next regularly scheduled IBC meeting. Deadline and meeting schedules may be found on
the Committee web page. IBC approval
must be obtained before using biohazardous materials. Once approved, the Committee will return a signed
approval memo back to the researcher via email.
All forms can be found on the Committee website.
6.B.1. Recombinant or Synthetic Nucleic Acid
Molecules
The following table summarizes experiments and
the required level of review. See the NIH
Guidelines (https://osp.od.nih.gov/biotechnology/nih-guidelines/) for more information.
|
Level of Review Required |
Type of Experiment |
|
NIH Director, RAC, IBC |
A drug resistant gene transferred into a (new) microorganism. (NIH Section III-A) |
|
NIH/OBA, IBC |
The cloning of toxin molecules with LD50 < 100 ng/kg of body weight. (NIH Section III-B) |
|
RAC, IRB, IBC |
Recombinant nucleic acid molecules, or DNA or RNA derived from recombinant nucleic acid molecules transferred into humans.Synthetic nucleic acid molecules, or DNA or RNA derived from synthetic nucleic acid molecules transferred into humans, that meet any one of the following criteria: (1) Contains more than 100 nucleotides; (2) Possesses biological properties that enable integration into the genome (e.g., cis elements involved in integration); (3) Have the potential to replicate in a cell; (4) Can be translated or transcribed. (NIH Section III-C-1) |
|
IBC |
Recombinant or synthetic nucleic acid molecules transferred to or from whole animals, whole plants, transgenic rodents, experiments involving >10 Liters of culture, at the appropriate Biological Safety Level (BSL). (NIH Section III-D) |
|
IBC |
Recombinant or synthetic nucleic acid molecules involving no more than 2/3 eukaryotic virus agents, whole plants, arthropods, or transgenic rodents. (NIH Section III-E) |
|
IBC |
Recombinant or synthetic nucleic acid molecules not found in organisms or viruses, single monochromal or viral DNA sources, or host DNA transferred to the same host or related species. (NIH Section III-F) |
6.B.2. Infectious Agents
The IBC also reviews work with biohazardous
agents including virus and bacteria.
6.B.2.a. CDC/NIH Requirements
Under the CDC/NIH guidelines in the BMBL, the principal
investigator must:
• Limit or restrict access to the laboratory
when work with infectious agents is in progress. The PI must include a
determination of who may be at increased risk and appropriately limit or deny
access.
• Establish policies and procedures to limit
access to those individuals who have been advised of the potential hazards and
meet specific entry requirements (e.g., immunization).
• Ensure that laboratory personnel are offered,
at no cost, appropriate immunizations or tests for the infectious agents
handled or potentially present in the laboratory (e.g., hepatitis B vaccine,
tuberculosis skin testing).
• Select and provide appropriate personal
protective equipment required for work with biohazardous materials.
• Ensure that laboratory and support personnel
receive appropriate training on the potential hazards associated with the work
involved, the necessary precautions to prevent exposures, the exposure
evaluation procedures, and that personnel receive annual updates or additional
training as necessary for procedural or policy changes.
• Develop standard operating procedures
incorporating biosafety procedures or a biosafety manual prepared specifically
for the laboratory, advise personnel of special hazards, and require them to
read and follow instructions on practices and procedures.
6.B.2.b. Local Requirement
for Standard Operating Procedures
For
projects including infectious agents and certain viral vectors, a set of
standard operating procedures is required.
Researchers may develop their own using a template the Committee
developed. See Appendix B.
6.B.3. Biotoxins
Biological toxins can include metabolites of
living organisms, degradation products of dead organisms, and materials
rendered toxic by the metabolic activity of microorganisms. Some toxins can also be produced by bacterial
or fungal fermentation, by the use of recombinant and synthetic nucleic acid
molecule technology, or by chemical syntheses of low molecular weight
toxins. Protocols utilizing biotoxins must be
reviewed by the IBC prior to use.
For more information and a list of
biotoxins refer to https://emergency.cdc.gov/agent/agentlist.asp.
6.B.4. Select Agents and Toxins
Currently there is no use of select
agents at the University of Vermont that falls under the federal regulations. For more information and a list of select
agents please go to https://www.selectagents.gov/ and https://www.selectagents.gov/SelectAgentsandToxinsList.html . If you intend
to use a select agent, please contact the Committee for further information
prior to obtaining the agent.
6.B.5. Human Gene Transfer Trials (NIH Appendix M)
Researchers planning a human gene transfer protocol should note that
there is a special review process for this type of work. All human gene transfer research protocols
must undergo review by the Recombinant DNA Advisory Committee (RAC) of the NIH
Office of Biotechnology Activities (NIH/OBA).
The RAC determination on the protocol must be obtained PRIOR to the
protocol receiving local IBC approval. IBC
approval needs to be obtained prior to human subjects review by the
University’s Institutional Review Board (IRB).
No reseach participant may be enrolled in a human gene transfer protocol
until the RAC review process is complete AND IBC and IRB approvals and
applicable regulatory authorizations are obtained. Furthermore, investigators may be required to
submit specific additional materials to NIH OBA prior to the enrollment of any
research participant. The industry
sponsor should make researchers aware of their obligations in this regard.
Researchers should inform the Research Protections Office as soon as
possible when considering submission of a human gene transfer protocol to
RAC. The office will attempt to conduct
simultaneous reviews with RAC, however no final determinations will be made
until the RAC outcome is known.
The IBC requires the following materials for review of a gene transfer
protocol:
1)
IBC Protocol Form,
2)
Clinical Protocol including tables, figures, and
relevant manuscripts,
3)
Investigational Drug Brochure,
4)
Responses to NIH Guidelines Appendices M-II through
M-V,
5)
Human Subject Common Protocol Cover Form,
6)
Informed consent draft, and
7)
Recombinant Advisory Committee (RAC) review (if
complete).
6.B.6. Animals and Recombinant or
Synthetic Nucleic Acid Molecules (NIH Appendix Q)
Recombinant and synthetic nucleic acid moleculeprotocols
which involve animals require review by the IBC and the Institutional Animal
Care and Use Committee (IACUC) committees.
The office will attempt to conduct simultaneous reviews with the IBC and
IACUC committees. To protect animals,
the IACUC approval will not be released until IBC approval has been
obtained.
The IBC requires the following materials for review
of research involving animals:
1)
IBC Protocol Form,
2)
Standard Operating Procedure (infectious agents and
viral mediated work)
6.B.7. Projects Involving Plants (NIH Appendix P)
Projects involving plants require review by the
IBC committee. Consultants may be called
upon to address these types of protocols.
The IBC requires the following materials for
review of this type of research:
1) IBC
Protocol Form
2) Standard Operating Procedure (infectious agents and viral mediated
work)
6.C. Revisions To Approved Projects
Principal investigators revising a currently
approved project must complete an Amendment form, revise appropriate protocol
pages, and submit one copy of each to the IBC for approval. Changes involving
modification of biological agents, significant procedure changes (including
change of the responsible principal investigator), changes to personnel, or
changes that increase the risk of the project and/or the biosafety level must
be approved by the IBC prior to implementing the changes.
Once approved, the Committee will return a
signed approval memo back to the principal investigator.
NOTE:
If the amendment involves vertebrate animals or human subjects, additional
review by other committees may be required prior to implementation.
6.D.
Continuing Review of Approved Projects
Annually, the Committee will forward to the
principal investigator a Continuing Review Form which must be completed and returned
to the Committee for review and continued approval.
Once approved, the Committee will return a
signed approval memo to the principal investigator.
Note:
The IBC may require an Investigator to complete a new IBC Protocol form
when protocols continue for extended periods of time or if the version of the
form template has changed significantly (e.g., substantive changes to the
questions or complete reorganization of information).
6.E. Reporting
6.E.1. Laboratory
Accidents and Exposures
All biological
exposures (i.e., life-threatening events), illness, or significant accident
leading to, or potentially leading to illness or that is environmentally
dangerous to humans and/or animals must be reported to the IBC as soon as
possible utilizing the Incident form, which can be found in the IBC forms library.
The
Chair and Biosafety Officer reviews all reports of biological exposures. All incidents will be reported to the IBC at
a regularly convened meeting at which time the IBC may require additional
safeguards or changes in procedures.
If
a biological exposure results in an infection, a full IBC, and if applicable, additional
research committee meetings, will be convened to discuss the incident, and all
biosafety procedures associated with the event.
In some instances the Chair of the IBC may suspend all relevant biohazardous
materials use by the PI pending clearance from the IBC and consultation with
medical specialists.
The
IBC will provide information about the reported event to the Office of Animal
Care Management and the Institutional Animal Care and Use Committee (when
applicable), and the Institutional Review Board (when applicable).
6.E.2. Additional Reporting
for Protocols that Involve Recombinant and Certain Types of Synthetic Nucleic
Acid Molecules
The NIH Guidelines
specifically require the reporting of significant problems, violations of the
NIH Guidelines, or any significant research-related accident or illness by the
Institution, the Institutional Biosafety Committee, or the Principal
Investigator.
The
Institutional Official will report in writing incidents that involve
recombinant and synthetic nucleic acid molecules to:
Office
of Biotechnology Activities
National
Institutes of Health
6705
Rockledge Drive, Suite 750, MSC 7985
Bethesda,
MD 20892-7985 (20817 for non-USPS mail)
Phone:
301-496-9838
Fax:
301-496-9839
Following
recommendations from the IBC the Insitutional Official will inform external
agencies such as the CDC, local public health department, State agencies, and
funding sources about the incident and corrective actions.
6.F. Notice
of Termination
Principal investigators must notify the IBC
when a project is completed or no longer active.
7. REQUIREMENTS OF THE PRINCIPAL
INVESTIGATOR
The principal investigator is responsible for the
following:
· Ensuring proper training and oversight of the research team;
· Ensuring protocol adherence, and;
· Providing reports on the progress of the study.
7.A. Proper
Training and Oversight of the Research Team
The principal investigator is responsible for ensuring that the research team has appropriate training prior to and during the conduct of the study by:
·
Rewiewing
with all laboratory staff the protocols that describe the potential biohazards
and the precautions to be taken (e.g., hazards and risks, immunizations,
personal protective equipment required, decontamination, storage and disposal,
spill procedures). Instructing staff in aseptic techniques and in the biology
of the organisms used in the experiments so that the potential biohazards can
be understood and appreciated.
·
Faculty members, principal investigators and others
responsible for directly, or indirectly, supervising labs will support and
encourage a culture of safety and the use of best practices in laboratory
protocols and procedures. This includes communicating safety and health as a
core value, understanding the risks and requirements associated with the
laboratories they oversee, assuring that appropriate precautions are taken
against hazards and unsafe practices, that proper personal protective equipment
is made available to all personnel, that workplace equipment and machinery is
routinely maintained, that required medical surveillance of impacted employees
is conducted, that regular safety inspections are performed and documented, and
that students and employees receive job and hazard-specific safety training. (NOTE: This excerpt is
taken from the UVM Laboratory Health and Safety Policy)
·
Required
web-based and classroom training:
UVM has subscribed to the web-based training
program, Collaborative Institutional Training
Initiative (CITI). Personnel
working on BSL-1 protocols must complete BSL-1 Basic Course in CITI and
personnel working on BSL-2 protocols must complete the BSL-2 Basic Course in
CITI AS WELL as the classroom
training with EH&S.
While it is not required, the Committee encourages researchers to complete the
higher level BSL-2 training as it is more comprehensive and will meet the BSL-1
requirement. Other required trainings as applicable to your protocol,
include Animal Biosafety,
Nanotechnology, Select Agents and DURC. Web-based training is required and must
be completed every three years. Reminder letters will be sent to personnel as
their training expiration date nears. Reference the CITI Program Training page for additional information about
required training and to check training completions. If
working in BSL3 level containment, appropriate training must be sought by
contacting the Biosafety Officer.
NOTE: The IBC Committee will not approve
key personnel until this requirement has been met.)
·
Instructing
and training laboratory staff in the practices and techniques required to
ensure safety and the procedures for dealing with accidents.
·
Informing
laboratory staff of the reasons and provisions for any precautionary medical
practices advised or requested.
·
Supervising
the safety performance of the laboratory staff to ensure that the required
safety practices and techniques are employed.
·
Investigating
and reporting any significant problems pertaining to the operating and
implementation of containment practices and procedures in writing to the IBC,
NIH/OBA (as required), and/or other appropriate regulatory authorities.
·
Correcting
work errors and conditions that may result in the release of these materials.
·
Ensuring
the integrity of the biological and physical containment (biosafety level).
7.B. Protocol
Adherence
It is the principal investigator’s responsibility to ensure that the IBC-approved protocol is being followed at all times by the research team. This includes making sure that amendments are submitted for IBC review in a timely fashion and then once approved implemented by the research team.
8. COMMUNICATION WITH THE IBC
The IBC requires investigators to submit all
protocols and protocol-related submissions (e.g. amendments, key personnel
changes) via an email attachment, preferably in
portable document format (PDF). Investigators in turn can expect to receive
their IBC correspondence via email. This
change is a giant step forward and should result in less paperwork for the
investigators and the IBC staff.
We continue to require protocol submissions to
be signed by the Biosafety Officer and the PI.
We have identified a potential pitfall with this new process to be
confusion with document versions. We
must all be vigilant about making sure we are always working with the currently
approved version of the protocol and protocol roster. Please update your documents every time they
are submitted by completing the footer with the date of the submission as shown
below.

This date
footer is not automatic, therefore you must change it each time you
revise your protocol. You should not use
the automatic date feature as this will add further confusion by changing your
date every time you happen to open the document. Failure to update this protocol version date
may delay review of the submission.
All submissions need to be sent to the IBC@uvm.edu email box where new
submissions will be monitored and processed in the order they are received.
When you are in communication with the office, whether in writing, by telephone, fax or e-mail, you should have the following information available.
- IBC number, if assigned at the time of contact
- Principal investigator’s name
- Protocol title
- Date and type of submission (if applicable)
We can more readily assist you with this information.
8.A. Written Communication of IBC Decisions
Decisions made by the IBC will be communicated to the principal
investigator (or designee if provided) through a memorandum outlining the
approval status and/or concerns, questions and/or comments of the IBC.
The IBC Chair will convey one of the following four decisions in
writing to the principal investigator promptly after the meeting:
Approval,
Review Simultaneous or Exemption Determination
The principal investigator may begin the research study upon receipt of
the Approval Memo, Review Simultaneous Memo, or the Exemption Determination
Memo from the Chair.
Approval
Withheld Pending Stipulations/Clarifications
This designation means the protocol is recommended for approval by the IBC
pending the principal investigator’s satisfactory response to IBC questions and
making revisions to conform to IBC-directed stipulations. The principal investigator must provide a memorandum
responding to the IBC’s questions and stipulations. The memo should reference
the IBC number and the applicable revised protocol pages should be attached.
Tabled
This designation indicates that more substantive issues regarding the
protocol must be addressed.
Clarifications or necessary revisions are significant in nature. A memorandum outlining the issues is sent to
the investigator. Full committee review of the investigator’s response and
revised protocol is required prior to approval.
Disapproved
This designation indicates that the risks of the biohazardous material are
of such significance that the committee cannot approve the project. The
authority of the IBC to disapprove a study may not be overridden.
NOTE: The IBC has a 30, 60, 90 day
reminder system for all pending protocol items.
The investigator will be reminded of an outstanding IBC request for information
or modifications. If no response is
received, at the 120 day mark the protocol is withdrawn from the Committee’s
consideration. This ensures that changes
to protocols are handled in a timely fashion.
9. COMPLIANCE
OVERSIGHT AND CORRECTIVE ACTION
OVERVIEW
It
is the responsibility of the Institutional Biosafety Committee (IBC) to address
noncompliance with University policies and procedures, the NIH Guidelines for Research Involving Recombinant
or Synthetic Nucleic Acid Molecules, and the Biosafety in Microbiological and
Biomedical Laboratories (BMBL) Manual for research which uses biohazardous
materials within the institution. To
exercise this authority the IBC is empowered to inspect laboratories, procedure
areas, animal housing areas, and to sequester research or training records. The
IBC may receive reports via external complaints, internal complaints, Incident
Reports, random and directed site visits with Biosafety Risk Assessments, and
investigator or laboratory worker self-reporting. The IBC encourages faculty,
staff and/or students to report instances of noncompliance.
This
document describes the procedures for handling these matters. This policy is not all encompassing, and the
IBC reserves the right to use its discretion in individual cases.
DEFINITIONS
Noncompliance is defined as the
conduct of research in a manner that deviates from the approved protocol or
disregards or violates federal regulations and/or institutional policies.
Noncompliance may result from intended or inadvertent actions or omissions by
study personnel, and can range from relatively minor or technical deviations to
serious deviations that threaten the safety of personnel or the
environment.
Serious
Noncompliance
is defined as noncompliance that, in the judgement of the IBC, potentially
increases the risk of harm to personnel or the environment.
Continuing
Noncompliance
is defined as a pattern of noncompliance (recurring or ongoing) that, in the
judgement of the IBC, may indicate an underlying deficiency in knowledge of the
regulations or IBC requirements or an unwillingness or inability to comply with
these regulations/requirements.
General
Noncompliance Review Procedures
The
investigation of potential noncompliance begins when the IBC becomes aware of
potential noncompliance. This may include an allegation (unproved assertion) of
noncompliance, a self-disclosure of noncompliance, or any other indication that
noncompliance may have occurred. The process
for the review of potential noncompliance involves initial administrative
review, followed by an inquiry/fact finding process if indicated. Once complete, the IBC makes a determination
as to whether the noncompliance is serious, continuing, or neither. The IBC
determination will be documented in a summary report that contains a corrective
action plan in cases of serious or continuing noncompliance. This process is
detailed below, however at any point in the review process, the IBC Chair or
Associate Chair, University Biosafety Officer, University Veterinarian, RPO
Director or Assistant Directors, or another Institutional Representative
designee may at their discretion:
·
Recommend
intervention for the safety of personnel or the environment
·
Recommend
the suspension of research activities
·
Inform,
involve, and/or provide salient documents to the PI, members of the research
team, the Department Chair, Dean, legal counsel, or Institutional Officials, as
appropriate
·
Initiate
reporting per federal regulations
·
Initiate
a monitoring visit
·
Recommend
immediate corrective actions
Process
of Noncompliance Review and Determination
Initial Review of Allegation or Indication of
Noncompliance: When
there is an allegation or indication of noncompliance, the first step is an
administrative review to determine if, in the judgement of the person(s)
conducting the review, there is the potential for serious or continuing
noncompliance. The initial review may be conducted by the RPO Director, RPO
Assistant Director(s), an IBC Chair (Associate Chair or Chair), University
Biosafety Officer, University Veterinarian or another Institutional
Representative. Allegations/indications which are determined to have no
potential to be serious and/or continuing noncompliance are resolved with
either no follow-up (i.e. when an allegation or indication has no merit) or
directly with the PI.
Inquiry/Fact Finding
Process:
If it is determined that the noncompliance has the potential to be
serious or continuing or if questions remain following the initial review, then
an inquiry (fact finding) process will begin.
The particular circumstances of the noncompliance will determine when
the fact finding begins and when the committee is briefed. The fact finding may
be conducted by any IBC designee including a sub-committee or subcommittee
member, the RPO Director, Assistant Director(s), an IBC Chair (Associate Chair
or Chair) or other Institutional Representatives. The IBC may be briefed at any
point throughout the fact finding process, as deemed appropriate by the
designee. The fact finding process continues until the designee has arrived at
a recommendation of determination (i.e. serious noncompliance and/or continuing
noncompliance, or neither). A fact finding report is then prepared and includes
the recommendation of determination and draft corrective actions. This fact finding report will be shared with
the PI, and if applicable, other person(s) involved. All parties will be provided an opportunity
to respond to any factual inaccuracies within the report before the committee
deliberates.
Deliberation by the
IBC:
At a convened meeting, the IBC will consider all available information and make
a determination as to whether the fact finding revealed serious noncompliance
and/or continuing noncompliance, or neither. The following factors will be
taken into consideration by the IBC or designee in making their initial
determination as to whether the noncompliance is serious and/or continuing
noncompliance. As each situation is unique, the indicators of noncompliance
that are important in one case may not be relevant in other cases.
Factors in the Determination of Serious
Noncompliance:
·
Level
of risk or potential risk to personnel or the environment
·
Severity
of safety violation
·
Frequency
or number of minor deviations or errors
·
Intent
·
Threat
to integrity of the IBC review processes and requirements for the protection
of personnel or the environment (i.e.
falsification of IBC documents)
·
Other
factors that, in the judgement of the IBC or designee, are relevant to the
situation being reviewed.
Factors in the Determination of Continuing
Noncompliance:
·
Similarity
of noncompliance to previous deviations and/or noncompliance within the same
registration or across registrations if the principal investigator has more
than one registration..
·
Likelihood
that instances of noncompliance will continue without intervention
Final Determination of
the IBC: If, in the judgement of the committee, the
noncompliance is neither serious nor continuing, this determination will
be shared with the PI. If, in the judgement of the committee, the noncompliance
is serious and/or continuing. The
designee will prepare a summary report including the IBC’s determination and an
approved corrective action plan. This
report will be shared with the PI, who will be given 14 days to review it
before it becomes final.
Development of Corrective Action Plans:
The IBC/Biosafety Officer/designee will develop
a proposed plan for corrective actions based on the information gathered during
fact-finding and input from the principal investigator and/or other affected
individuals. The proposed plan may:
·
Require
no further action
·
Require
minor corrective actions to achieve compliance
·
Require
additional education
·
Require
the investigator and/or other affected individuals to develop and implement
procedures to prevent recurrence
·
Review
internal departmental or institutional mechanisms and systems for opportunities
to prevent recurrence or similar occurrences by others
·
Require
additional oversight (e.g., by other faculty member or department process)
·
Require
more frequent IBC reviews
·
Require
internal monitoring visits or monitoring plans
·
Suspend
or terminate individual protocols
·
Restrict
researcher’s research activities
REQUESTS
FOR RECONSIDERATION
A
PI may request a reconsideration of the IBC’s determination. Requests must be
limited to claims that either (1) the process was faulty, resulting in
considerable risk that the outcome was incorrect; or (2) that the findings
and/or corrective actions imposed by the IBC were excessive or
unjustified. The written request must be
submitted within 14 days of receipt of the summary report and must specify the
nature of any claimed procedural error or the perceived unfairness of actions
taken. Reconsiderations will be conducted by an IBC Chair (Chair or Associate
Chair), Biosafety Officer, or Designee.
The reconsideration process will result in one of three outcomes, either
the summary report will stand and it will become final, the summary report will
be modified and become final, or further investigation is necessary and will be
initiated.
Required
reporting
When
noncompliance is determined to be serious and/or continuing, the final report
will be forwarded to federal regulators if required, and to applicable
Institutional Officials, the Departmental Chair, the Dean, and sponsors, if
applicable.
Guiding
Principles for NonCompliance Review
Protection
of Personnel and the Environment: The University of
Vermont is committed to minimizing the
risks to faculty, staff, students, the public, the facilities, and the
environment while using biohazardous materials during research at UVM.
Fairness: The IBC strives to maintain a review that is
impartial and honest, free from self-interest, prejudice or favoritism,
including member recusal if such a self-reported or identified conflict
arises.
Communication: The committee will
communicate with the PI during the review process at points determined to be
appropriate by the IBC designee.
Confidentiality: All IBC discussions
and documents regarding a situation of noncompliance are considered sensitive
and will be handled in a confidential manner and in accordance with state and
federal regulations. The IBC cannot,
however, guarantee complete anonymity to informants or witnesses. Confidentiality will be maintained to the
extent possible to protect privacy and prevent retaliation, while still
allowing for a full and fair review. Information may be shared, as described
above under Required Reporting.
Conflict of Interest: Any IBC member who feels that they have a
conflicting interest must recuse themselves from reviewing the issue of
noncompliance. IBC members who are also
listed as key personnel on the protocol(s) will not participate in the review
but may be asked for information.
Procedures: In addition to what has been stated within
this policy, the Committee will follow all applicable procedures that are
outlined within the Committee Operating Procedures document.
10. Post Approval Monitoring
In order to assist the UVM research community in adopting
best laboratory Biosafety practices that help ensure a safe laboratory
environment, the UVM Institutional Biosafety Committee has adopted the practice
of regularly assessing laboratories associated with an active IBC
protocol. Assessments are an opportunity
for laboratory personnel to receive guidance on prudent laboratory/Biosafety
practices and procedures, ask questions and voice concerns. This process is in
addition to regular risk assessments conducted by the UVM Biosafety Officer in
support of new protocols and amendments to existing protocols.
Assessment Frequency
Assessment
frequency will depend upon the level of risk associated with the laboratory
work and the principal investigators history of compliance/non-compliance with
IBC policies. In the absence of any IBC related violations, the UVM IBC will
adhere to the following assessment schedule:
This schedule
includes teaching laboratories.
BSL-1
laboratories – every 3 years
BSL-2
laboratories – every 2 years
BSL-3
laboratories – annually
Appendix A - How to Determine Risk Group and Biosafety Level Containment
The
following tools can help researchers make an initial determination of the
appropriate risk group and containment level and practices. The Biosafety Officer, after collecting the
details necessary at a lab site visit, will bring his recommendation to the
full Committee who will make the final risk group and containment level
determination.
Follows
is a link to the NIH/CDC BMBL 5th edition table to help get you
started
http://www.cdc.gov/biosafety/publications/bmbl5/index.htm
The
American Biological Society Association maintains an excellent reference for
risk groups at https://my.absa.org/Riskgroups
Appendix B –
Template SOPs
The
IBC Committee has developed a standard template standard operating procedures (SOPs)
for your use. The template can be downloaded
from the IBC forms
page.
Attachment
C – IBC Forms
All forms and form
instructions are located in the forms section of our website and should be
downloaded each time you need one. (SEE: http://www.uvm.edu/ibc and click on “Forms”)
Attachment
D – Exposure Control Plan for Bloodborne Pathogens
UVM’s Risk Management and
Safety Office has an appropriate bloodborne pathogen control plan which is
located at http://esf.uvm.edu/uvmecp/.