In
order to make the best use of this tutorial, keep two windows open
on your Desktop!
-
In the first window, view this
tutorial. Even though you may have a hard copy, you can't follow the links
unless you have this page open in your browser.
-
In the second window, run Protein
Explorer with the DNA model.
|
To see what CHIME can do, first
open
a new window. In the new window, go to: "
MolViZ.Org. Molecular Visualization Resources"
(hosted on the San Diego Supercomputer) .
Then click on
the link to "
Hemoglobin".
The index page
for the Hemoglobin tutorial should appear. Click on the Hemoglobin
link in large bold text. Now you should see a black
page with 2 frames. The right frame contains links to 3D visualizations
of the various aspects of hemoglobin structure which will be explored.
In the right frame of the Hemoglobin Index page, click on "Hemoglobin
& Heme".

Hemoglobin and Heme
Click on
image to see full-size! |
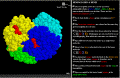
 The
hemoglobin molecule is four polypeptide chains .... The
hemoglobin molecule is four polypeptide chains ....
Click on the "X" button opposite
"The hemoglobin molecule is made up of four polypeptide chains ....".
This should load a rotating model of the hemoglobin molecule in the left
frame. The rotating model of hemoglobin shows several things:
-
It is composed of four separate chains of amino acids (polypeptides).
Each polypeptide is displayed in a different color.
-
Each polypeptide binds one heme group which is displayed in red.
|
|
Click on
image to see full-size! |
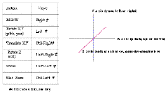
Viewing
the molecule:
-
Translate X or Y (moved left or right; up or down).
with the mouse by holding down the control key and the right
button on the mouse.
-
Rotate. The image can be yawed
(rotated left and right about the Y axis ) or pitched (rotated up or down
about the X axis) with the mouse by holding down the left button
on the mouse.
-
Roll. The image can be rolled
about the Z axis with the mouse by holding down the shift key and
the right button of the mouse.
Zoom. The image can be resized
with the mouse by holding down the shift key and the left button
on the mouse.
|
|
Click on
image to see full-size! |
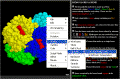
Display
Menu.
A menu for changing the display is
accessed by holding down the right button of the mouse
-
Rotation: This function can be
toggled on or off.
-
Display: The "Display"
menu allows us to view different aspects of the structure:
-
Hold down the right mouse button. Choose "Display" --- "Spacefill"
--- "Van der Waals Radii".
-
Resize the molecule by holding down the shift key and the left mouse button.
Move the mouse up or down to zoom in or out. Reduce the molecule
to the height of your window.
-
Manipulate the molecule with your mouse to stand it up vertically.
-
Hold down the right mouse button. Choose "Options" --- "Rotation".
-
Click on "X Spin".
-
Hold down the right mouse button. Choose "Options" --- "Stereo Display".
-
Reset:
Click on the "X" button opposite "The hemoglobin molecule is made up of
four polypeptide chains ...."
|
|

Click on
image to see full-size! |
Color
Menu.
The following options are available for color display. Play with
them.
-
Monochrome - self explanatory
-
CPK (carbon grey; hydrogen white; oxygen red; nitrogen blue; phosphorus
gold)
-
Amino Acid - every amino acid is displayed in a different color
-
Shapely - the amino acids are displayed according to the chemical
properties of their side chains
-
Group - this emphasizes the different elements of secondary structure
and the ends of each polypeptide chain. It is most useful when used
in the "ribbon" or "cartoon" display mode.
-
Chain - each of the polypeptide chains is displayed in its own color
-
Structure - this emphasizes the different secondary structures.
Alpha helix is displayed in rose; beta pleated sheet in gold; random coil
in thin blue/white lines. It is most useful when used in the "ribbon"
or "cartoon" display mode.
Temperature and User: not useful for our purposes.
|
|
Click on
image to see full-size! |
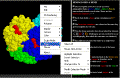
The
Select
Menu displays different parts of the molecule in different ways.
We know that proteins have shape because it is necessary for
them to bind something in order to perform their function.
For example and enzyme has an active site into which the substrate
fits. More generally we refer to these as the binding
site on the protein and the ligand
which fits into it.
In Hemoglobin we can display the protein chains and the ligand (the
Heme group) in different ways. We can display hemoglobin to
emphasize the shape of the binding site:
-
Hold the mouse button down. Go to Select; then go to Hetero;
then go to Ligand ("hetero" refers to any other atoms and
molecules which are not part of the polypeptide chains).
-
Go to the Color menu and select "CPK"
-
Go back to Select; then go to Protein.
-
go to the Display menu and select "Wireframe"
-
You should now see something like this.
Move one of the Heme groups to the center of the screen. Zoom
in; note how the atoms in the side chains of the amino acids closely
contact the Heme group. They form a binding site which holds the
Heme group in. Now
you can see why SHAPE is so important!!!! |
|
| The other buttons in this section
of the tutorial:
 Each chain holds
a heme group containing one Fe++ atom. Each chain holds
a heme group containing one Fe++ atom.
 The heme-iron complexes
are colored red because they give hemoglobin its red color. Actually,
it is the yellow colored atom in the center which gives the Heme group
the red color. This atom is Iron. Heme has a red color because
oxidized iron (rust) is red! This is also why oxygenated blood
is red. The heme-iron complexes
are colored red because they give hemoglobin its red color. Actually,
it is the yellow colored atom in the center which gives the Heme group
the red color. This atom is Iron. Heme has a red color because
oxidized iron (rust) is red! This is also why oxygenated blood
is red.
 Now the heme molecules
have been colored by element. The Heme groups displayed using
CPK colors. Now the heme molecules
have been colored by element. The Heme groups displayed using
CPK colors.
 Spacefill view of
atoms that make up a single heme molecule. The Heme group displayed
to show the true size of each atom, and the real shape of the molecule. Spacefill view of
atoms that make up a single heme molecule. The Heme group displayed
to show the true size of each atom, and the real shape of the molecule.
 Here is how iron
is attached to the rest of the heme molecule. Here is how iron
is attached to the rest of the heme molecule.
 An elemental oxygen
molecule binds to the ferrous iron atom in the lungs where oxygen is abundant,
and is released later in tissues which need oxygen. Note that there
is a difference between a single oxygen atom and an Oxygen molecule
which is composed of two oxygen atoms! An elemental oxygen
molecule binds to the ferrous iron atom in the lungs where oxygen is abundant,
and is released later in tissues which need oxygen. Note that there
is a difference between a single oxygen atom and an Oxygen molecule
which is composed of two oxygen atoms!
 The position of bound
elemental oxygen in one chain of hemoglobin. The position of bound
elemental oxygen in one chain of hemoglobin.
 Space occupied by
the heme bound oxygen in the polypeptide chain. Space occupied by
the heme bound oxygen in the polypeptide chain.
 A histidine nitrogen
binds to the iron, helping to anchor its position. Don't worry about
this one! A histidine nitrogen
binds to the iron, helping to anchor its position. Don't worry about
this one!
 A spacefill view
(with the exception of the heme molecule) of the hemoglobin polypeptide
chain. This view shows a stick model of the Heme group with the
yellow colored iron atom in the center. The molecules on either side
displayed in spacefill mode and CPK color are the side chains of 2 amino
acids in the protein chain. A spacefill view
(with the exception of the heme molecule) of the hemoglobin polypeptide
chain. This view shows a stick model of the Heme group with the
yellow colored iron atom in the center. The molecules on either side
displayed in spacefill mode and CPK color are the side chains of 2 amino
acids in the protein chain.
-
Hold down the button on your mouse and go to the Options menu.
At the bottom choose Stereo Display. Adjust the size to look
something like this.
Put your nose close up to the screen, and move slowly backward concentrating
on the "third" image in the center. At some point the image should
appear 3 dimensional! This view gives you a good idea of how closely
ligands fit into binding pockets .......... and how important SHAPE
is to FUNCTION!!!
|

Secondary Structure
At the bottom of the HEMOGLOBIN & HEME tutorial is a "Back"
button. This will return you to the first
page. Click on the link for "Hemoglobin Secondary Structure".
 Most of the amino acids
in hemoglobin form alpha helices. Most of the amino acids
in hemoglobin form alpha helices.
When you click on the "X" button, the rotating model of the hemoglobin
molecule will load again. Stop the rotation so you can view the
molecule easily. What you see is one of the four polypeptides
and a single heme group (the author does not specify whether this
is an a or b globin
chain).
| REMEMBER from
the tutorial above that: "The hemoglobin molecule is made up of four
polypeptide chains (Alpha 1, Beta 1 , Alpha 2, Beta 2), non-covalently
bound to each other. There are four heme-iron complexes." |
The segments of the amino acid chain which fold into a
helix
are shown in red. Random coil (the segments which do not fold into
a
helix)
are shown in white. Start the rotation again so you can
see the overall 3D structure.
 A rainbow coloring
scheme from the N-terminus to the C-terminus helps to discern the separate
alpha helices. A rainbow coloring
scheme from the N-terminus to the C-terminus helps to discern the separate
alpha helices.
This coloring scheme helps to trace out the chain from the beginning
which is colored blue -- light blue -- teal -- green -- yellow -- orange
-- red, which is the end.
-
Where is the a helix?
-
Where is the Random Coil?
 This is a cartoon representation. This is a cartoon representation.
-
Where is the a helix?
-
Where is the Random Coil?
 We'll focus on a single
alpha helix. This helix is at the protein-water interface. We'll focus on a single
alpha helix. This helix is at the protein-water interface.
 Here is the isolated
alpha helix. Here is the isolated
alpha helix.
 The backbone representation
connects alpha carbon positions in this alpha helix. These lines do not
represent the positions of any actual chemical bonds. The backbone representation
connects alpha carbon positions in this alpha helix. These lines do not
represent the positions of any actual chemical bonds.
 Here are the actual
bonds of the alpha helix backbone: three atom repeats of nitrogen, alpha
carbon, carboxy carbon. Here are the actual
bonds of the alpha helix backbone: three atom repeats of nitrogen, alpha
carbon, carboxy carbon.
Stop the rotation. Use the mouse to manipulate the molecule into
a vertical position. Reposition the model to the center of your window.
Resize the model so that the entire length fits in your window. Using
the mouse menu go to Color; then Amino Acid. Now you
can see the individual amino acids in the chain which is coiled into the
a
helix.
-
How many amino acids are in this short
polypeptide?
Using the mouse menu go to Options; then Stereo Display.
Start the rotation. Now you can see exactly how the amino acid chain
coils to form this secondary structure.
 Hydrogen bonds (white)
stabilize the alpha helix. Don't worry about this one.
View it if you are interested. Hydrogen bonds (white)
stabilize the alpha helix. Don't worry about this one.
View it if you are interested.
 The sidechains on
the alpha carbons are shown. Don't worry about this one.
View it if you are interested. The sidechains on
the alpha carbons are shown. Don't worry about this one.
View it if you are interested.
 Now the sidechain
elements are identified: C H O N Don't worry about this
one. View it if you are interested. Now the sidechain
elements are identified: C H O N Don't worry about this
one. View it if you are interested.
|
 Send
us questions or comments!
Send
us questions or comments!
![]()