Click
on thumbnail to see full-size! |
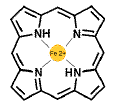
The
Porphyrin Ring chelates an Iron atom. Porphyrins are widely distributed
in nature. They occur in hemoglobin and myoglobin. However they are also
found in chlorophylls (the green pigments in plant which trap light energy),
peroxidases, Vitamin B12, and the cytochromes of the electron transport
chain.
The essential characteristic of the
porphyrin ring is the ability to chelate a number of metals such as iron
(Fe), copper (Cu), zinc (Zn), nickel (Ni), or magnesium (Mg) in
the case of chlorophyll.
In myoglobin and hemoglobin, there
are 4 heterocyclic nitrogens which bind the iron atom.
Another important characteristic
is that - because of its aromaticity - the porphyrin is very hydrophobic! |
|
Click
on thumbnail to see full-size! |
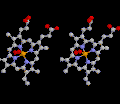
The
Iron atom binds molecular oxygen (O2).
Iron and Copper are readily oxidized to iron oxides which have a red color.
This is what make a-globin,
b-globin
and myoglobin good oxygen carriers.
The thumbnail to the left shows how
molecular oxygen is bound is bound to one side of the Fe2+ atom.
Unfortunately, carbon monoxide binds to the iron even more strongly than
oxygen. This is why carbon monoxide is so toxic - it removes oxygen from
the blood stream and asphyxiates the individual.
| In order to view the stereo image in 3-D, look at it from a distance
which is so close that you can't focus. At this point, you should see 3
images.
Then slowly move out, trying to focus on the third image in the center.
When this is brought into focus, it will appear 3-dimensional. |
|
|
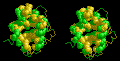
Click
on thumbnail to see full-size! |
The
tertiary structure of myoglobin forms a binding pocket for the Porphyrin
Ring. Myoglobin is
composed of 8 alpha-helices, which fold on each other to form a hydrophilic
exterior (shown in green) and a hydrophobic interior (yellow). A cleft
in the protein gives access to this hydrophobic interior.
Recall that the porphyrin ring
is very hydrophobic. It can "escape" from the aqueous environment of
the cells and tissues by binding into the cleft of myoglobin!
|
|

Click
on thumbnail to see full-size! |
A
stereo space-filling view of the Myoglobin binding pocket. The thumbnail
to the left shows a space-filling stereo view of myoglobin. This shows
the real shape of the binding pocket. |
|
Click
on thumbnail to see full-size! |
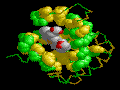
A
Porphyrin Ring in the Myoglobin binding pocket. The thumbnail to the
left shows the polarity of the alpha helices, with the porphyrin ring inserted
into the binding pocket. |
|
Click
on thumbnail to see full-size! |
A
stereo space-filling view of the Porphyrin Ring in the Myoglobin binding
pocket. The thumbnail to the left shows a space-filling model, with
the porphyrin ring inserted into the binding pocket. |
|