Often these atoms are depicted with only their unpaired electrons. The paired electrons in the filled orbitals are not shown to make the diagram simpler. Each would then appear as below:
The study of biology requires an understanding of simple organic chemistry and simple biological chemistry. Carbohydrates, lipids, proteins, and nucleic acids, the players in biology, are themselves composed of smaller building blocks. This chapter contains a review of important chemical interactions and concepts you will encounter in this course.
Carbon has 4 unpaired electrons and 4 unfilled orbitals; nitrogen
has 3; oxygen has 2; and hydrogen has 1 unpaired electron
and 1 unfilled orbital :
Often these atoms are depicted with only their unpaired electrons.
The paired electrons in the filled orbitals are not shown to make the diagram
simpler. Each would then appear as below:
| w |
One of the simplest molecules is water
which has 2 covalent bonds:
| In this figure, oxygen has 2 unpaired electrons (shown in red), and 2 unfilled orbitals. The 2 hydrogens each have 1 unpaired electron (shown in gold), and 1 unfilled orbital. By sharing the 4 unpaired electrons between them, each atom can have a filled outer orbital with paired electrons. The result is two covalent bonds. |
There are 2 ways of depicting the covalent bonds which are formed by
sharing of atoms. One way shows the atoms which are being shared
(as in the graphic of water above). The other way shows the covalent
bonds only - in effect each pair of electrons
is indicated by a line which represents the covalent bond.
The figure below shows the same Carboxylic
Acid molecule depicted in each of these 2 ways. The carboxyl
group is COOH. When a carboxyl group is added to any chemical
"R" (shown in green), a carboxylic acid is formed.
| This shows each of the covalent bonds represented as a line. Each line represents a pair of shared electrons. From Organic Chemistry you will remember that the oxygen which forms the double bonds is referred to as a keto oxygen. The O-H group is referred to as a hydroxyl group. When a carboxylic acid is dissolved in an aqueous solution the hydrogen atom (gold) ionizes , leaving as a proton (H+) which increases the acidity and lowers the pHof the solution. | |
| The carbon atom has 4 unpaired electrons. One of the carbon electrons (shown in black) is shared with an unpaired electron (shown in green) from the unspecified chemical "R" . The keto oxygen forms a double bond to the carbon. In this case both of the unpaired electrons from the oxygen (shown in red) are shared with two unpaired electrons (black) from the carbon. The second oxygen shares one of its unpaired electrons (red) with the fourth unpaired electron of the carbon (black). This results in a single C - O covalent bond. The second unpaired electron of the oxygen (red) is shared with the unpaired electron of the hydrogen (gold). This forms an O - H covalent bond. |
Covalent bonds are very strong - once formed, they rarely break spontaneously. Covalent bonds are much stronger than Ionic Bonds, Hydrogen Bonds or Van der Waals Forces.
There are single, double, and triple covalent bonds:
Bond Number Example H | single H--C--H | H H H | | double H--C==C--H | | H H H | C triple ||| C | H
| w |
Covalent bonds can also have partial charges when the atoms involved have different electronegativities. Water is perhaps the most obvious example of a molecule with partial charges. The symbols delta+ and delta- are used to indicate partial charges.
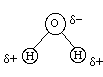 Oxygen
is very strongly electronegative (it has a strong "attraction"
for electrons). Conversely, Hydrogen is very weakly
electronegative. As a result when oxygen and hydrogen are bonded,
they share electrons - but they do
NOT
share
them equally! Statistically the electrons are thought
of as averaging more time closer to oxygen than to
hydrogen. Consequently oxygen has a partial negative charge
(it is not a FULL negative charge because the electrons do not spend 100%
of their time with oxygen; they still spend some of their time with hydrogen,
so the negative charge is only partial). Moreover, since the electron
spends less time in the vicinity of the hydrogen, the hydrogen carries
a partial positive charge.
Oxygen
is very strongly electronegative (it has a strong "attraction"
for electrons). Conversely, Hydrogen is very weakly
electronegative. As a result when oxygen and hydrogen are bonded,
they share electrons - but they do
NOT
share
them equally! Statistically the electrons are thought
of as averaging more time closer to oxygen than to
hydrogen. Consequently oxygen has a partial negative charge
(it is not a FULL negative charge because the electrons do not spend 100%
of their time with oxygen; they still spend some of their time with hydrogen,
so the negative charge is only partial). Moreover, since the electron
spends less time in the vicinity of the hydrogen, the hydrogen carries
a partial positive charge.
-------------------------------------------------------------------------------------------------------
The possibility of hydrogen bonds (H-bonds)
is a consequence of partial charges. The classical example of hydrogen
bonding is in water, as shown below. Hydrogen bonds are formed when
the partial positive charge on a hydrogen atom is attracted to
the partial negative charge on an oxygen atom.
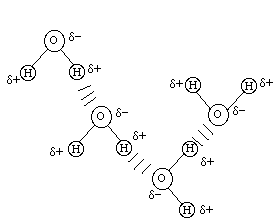
----------------------------------------------------------------------------------------------------------------------------
Nitrogen is also very electronegative and can participate in hydrogen bonding.
More examples: H | R--O--H ||| N--R R==N--H ||| O==R | Note that R stands for H any side group.
| w |
| w |
Remember: Covalent bonds are shared electrons. However there is no way to tell exactly where they are at any precise femto second (10-15 seconds) of time. Their location is only a matter of statistical probability. Another way of saying this is that on average they will exist in a certain "territory" between the two atoms which are sharing them - but one can never say exactly where they are at any instant - and on average they will be equally shared:
In the figure below, a Van der Waals bond is shown as the dashed line between two methane molecules.
Why
can Geckos walk on the ceiling?
| w |
AFTER YOU HAVE STUDIED THIS PAGE AND ALL LINKS:Proceed to "Bio-Organic Molecules" |
![]()
Go to the Top of the Page.
|
Site Map for Genetics |
| Why don't Oil and Water Mix? |
********* THIS IS ALL YOU ARE REQUIRED TO KNOW. PROCEED
FURTHER IF YOU WISH EDUCATION ONLY FOR ITS OWN MERITS! **************
To understand the energetics driving this interaction,
visualize
the H2O molecules surrounding droplets of "dissolved" hydrophobic
molecules. The water molecules attempt to form the greatest number
of hydrogen bonds with each other - however those which find themselves
at the surface of a hydrophobic droplet are blocked from forming
H-bonds with other water molecule, as shown below:
The best energetic solution involves the water molecules forcing all of the nonpolar molecules together, thus reducing the total surface area presented by the hydrophobic molecules which breaks up the H2O H-bond matrix.
The total amount of nonpolar material present is the same in both the
right and left diagrams. Because of the geometry of surface: volume
ratios however, the surface area presented by the
large droplet is far less than the surface area presented by the 9 small
ones. Therefore the number of water
molecules which can form hydrogen bonds with others is maximized when the
hydrophobic molecules are all aggregated together and the situation pictured
on the right is energetically favored.
This phenomenon is extremely important in determining
the secondary structure of proteins, particularly Random
Coil.
********
********
Look here for the contribution of Chemistry to Comic Books, and vice-versa.