Agrobacterium rhizogenes
transformation
This protocol uses the natural genetic engineer, Agrobacterium rhizogenes, to transfer genes to roots. This process is virtually identical to the way that its famous cousin, A. tumefaciens, transforms plants, with a few differences. First, the relevant plasmid in A. rhizogenes is called the Ri plasmid (rather than Ti in A. tumefaciens) for Root inducing. Second, the A. rhizogenes strain that we use still contains the virulent Ri plasmid. Most A. tumefaciens strains used for genetic engineering in labs, have the virulent Ti plasmid removed. Key functions are located on the binary plasmid, and essential vir genes are on a helper plasmid in the strain. All the tumor-inducing genes, however, are gone from lab A. tumefaciens strains. In contrast, all the root-inducing genes (so, things that mess up the hormone balance) are still there in the A. rhizogenes strain we use. That is why this process still works, but it also means that these roots are not exactly like wild-type roots…
There are lots of ways to transform roots using A. rhizogenes. Each lab seems to use a slightly different technique, and some are very different! This is the way that Ulrike Mathesius’s lab transforms Medicago. It works very well for them. They get a high rate of transformation, but they need to select, otherwise you get chimeric roots. You need to always check, because sometimes the lateral roots growing out of a transformed root are not transformed themselves! That’s why all their constructs have a constitutive GFP gene. Uli thought DsRed should work just as well, if not better. However, they can just check for GFP expression using a dissecting scope fitted with fluorescence. I’m not sure whether that is possible with DsRed, which is often not as bright.
Key to success!
Uli says the key to their success is two-fold. First, they pour their plates on a slant, so only the bottom half is filled with agar. This is important because they put the Kanamycin (their selectable marker) in the agar. Since chloroplasts are sensitive to antibiotics (they are derived from bacteria, after all!), plants tend to do better when the green parts of the plants do not come into direct contact with the medium. Second, Uli’s lab uses a particular brand of agar that is very cheap, but does the trick. They are the only lab I know of that can nodulate hairy roots on agar. Everyone else has to transfer the chimeric plants to either filter paper, turface or growth pouches. So probably the kind of agar they use is key. (Most people assume it is some kind of contaminant in the agar that inhibits nodulation in hairy roots.)
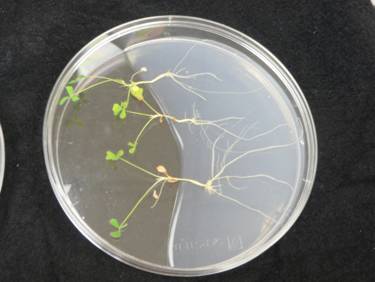
Usually they put more plants/plate, so that it looks like
this.
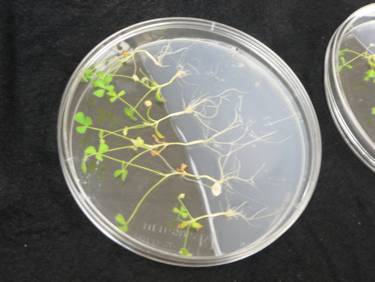
It doesn’t have to be round plates, but they are much cheaper than the large square plates! Also, these roots do not grow very long, just bushy. Uli sets the plates vertically, in a box, with pieces of black paper between the plates to shade the root.
The Protocol: A. rhizogenes transformation
1. Pour media into 15 cm round petri plates. Pour half plates (as shown above) by putting a little petri plate lid under one end of the plate to raise it slightly off the benchtop. Use Farhraeus medium containing selection (e.g. Kanamycin), and make sure to use special agar.
Company: Gelita Australia Pty Ltd. (old can said “Leiner Davis Gelatin Company” phone number was the same for both companies)
Telephone: (07) 5541 3544
Fax: (07) 5541 3612
Product:
Powdered Agar
Grade J3
10kg
2.
Germinate seedlings
overnight.
3. Cut off root tip. The seedlings should be about 1 cm long when you cut off the root tip. You are meant to cut 3 mm from the tip. Anton Wasson (Uli Mathesius’s lab) says he has successfully transformed with seedlings which have overgrown a little, up to 2 cm. He generally maintains proportions (i.e. if it is 2 cm long, cut 6 mm from the tip), but thinks the important thing is to cut off the meristem in the tip.
4. Dip wounded root tip into colony of A. rhizogenes containing plasmid with construct of interest. Anton says, “The cut end of the seedling is just lightly touched to the rhizogenes bacteria [containing your binary plasmid of interest] growing on a plate. Some people scrape the end through the bacterial growth, which I think harms the root too much.”
5. Place on Fahraeus medium in half plates so that shoots are on air and roots are on agar. Put in growth chamber set at 20°C. [note: Anton parafilms ¾ of the plate, leaving the bottom open. I wonder whether surgical tape would be an improvement?]
6.
After 1 week, trim off any adventitious roots that have started from the hypocotyl. In another
week they will be hard to distinguish from the transformed roots. This is
especially important if you do not have a secondary marker like GFP or DsRed.
Really transformed roots will not usually appear until the second week.
7.
Uli’s lab transfers plants to 25°C one week after treating with A. rhizogenes.
8.
Inoculate with Rhizobium one week after the transfer to 25°C. If roots still
look small, wait until they grow longer before inoculating.
Other factors Anton finds important: the strain of A. rhizogenes and the temperature. “A week at 20°C after
inoculation makes a big difference to overall efficiency of transformation.
Then you can put the plantlets at 25°. I tried four strains of rhizogenes before we went for the original ARQUA1. It seems to
work much better.”