The oxidation kinetics of ferrous iron are well known to be pH dependent, with the slow oxidation kinetics of ferrous iron at low pH being part of the early basis for considering the importance of microbial iron oxidation in the formation of acidic Mine drainage (Singer and Stumm, 1970). There are many known iron-oxidizing microorganisms in environments at circumneutral pH, where the abiotic oxidation of iron is fast enough that the microbes must effectively compete with the abiotic process. Additionally, microbes must compete with each other for the available ferrous iron as substrate, with organisms able to utilize the iron faster in a particular environment making up the predominant part of a community including iron oxidizers.
As part of a body of work started at the University of Delaware, and continuing at the University of Vermont, we are working with Dr. Dave Emerson at the American Type Culture Collection and George Mason University to characterize the cell-normalized rate at which circumneutral iron-oxidizing organisms utilize iron as a function of oxygen availability. This is coupled to field and laboratory work on identifying the geochemical niche certain organisms thrive in to determine how they outcompete abiotic processes and each other for dominance.
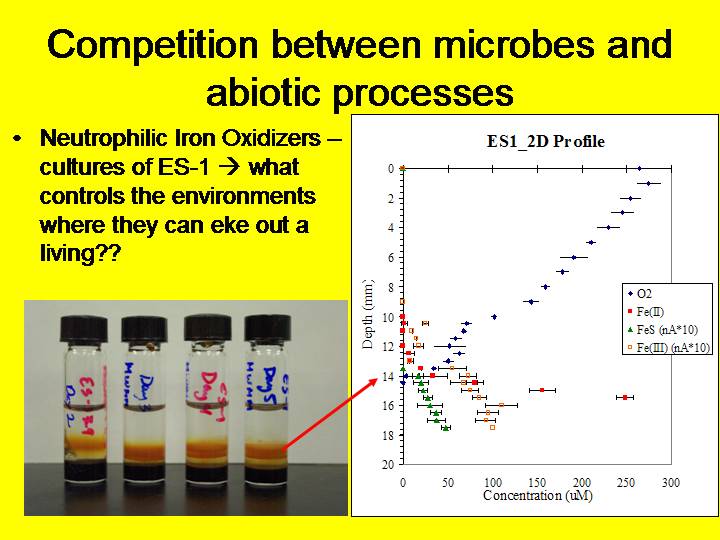
Figure - Gradient tubes of ES-1 an environmental isolate of a neutrophilic iron-oxidizing microbe
To define cell-normalized rates, we utilize electrochemical methods to observe the rate of Fe2+ reaction in cells where we control the partial pressure of oxygen. We do this with and without specific organisms and with kill controls in specific T, pH, and solution compositions. We also characterize the geochemical niche each organism utilizes through electrochemical characterization of the redox chemistry (O2, Fe2+, Fe3+, FeS(aq)) in gradient culture tubes.
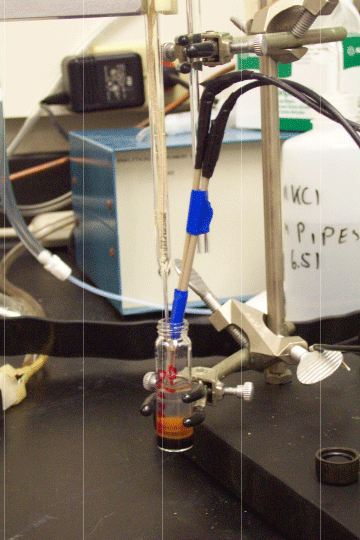
Figure - Use of a glass Au-amalgam micrelectrode to profile the environment this ES-1 isolate is thriving in.