Section I Committee Information
1. Committee Mission
The University of Vermont is committed to the humane care and use of animals in activities related to research, testing and teaching.
The Institution has an Assurance on file with the Office of Laboratory Animal Welfare (OLAW) in accordance with PHS Policy and has been an accredited through AAALAC International for many years.
2.0 Introduction to the Committees
In accordance with the Animal Welfare Act of 1966 and the Health Research Extension Act of 1985, the University of Vermont Institutional Animal Care and Use Committee (IACUC) serves as the University's central review body for matters relating to the care, use and treatment of animals in activities related to research, testing and teaching.
The IACUC Committee is charged with reviewing activities involving animals conducted at or sponsored by the University of Vermont in order to ensure that the animals are utilized in accordance with all pertaining Federal, State, municipal or other pertinent law.
The IACUC Policy and Procedure Committee, which includes Committee leadership, Veterinarian, and IACUC staff, convenes monthly to review changes in policy and procedures and new regulations.
Ad-hoc Noncompliance Subcommittees, including a subset of the members and other institutional personnel as applicable, convene as necessary to review noncompliance cases.
Guiding Principles
The University has adopted, on an institution-wide basis, the principles regarding animal care as stated in the Animal Welfare Act. The Animal Welfare Act was signed into law in 1966. It is the only Federal law in the United States that regulates the treatment of animals in research, exhibition, and transport. The Act is enforced by USDA and APHIS.
The OLAW Policy of the Public Health Service (PHS) requires institutions to establish and maintain proper measures to ensure the appropriate care and use of all animals involved in research, research training, and biological testing conducted or supported by the PHS. PHS requires institutions to use the Guide for the Care and Use of Laboratory Animals (PDF).;
The IACUC also references the Guide for the Care and Use of Agricultural Animals in Research and Teaching when caring for and using agricultural animals.
AAALAC International is a private, nonprofit organization that promotes the humane treatment of animals in science through voluntary accreditation and assessment programs. “When animals are used, AAALAC works with institutions and researchers to serve as a bridge between progress and animal well-being. This is done through AAALAC's voluntary accreditation process in which research programs demonstrate that they meet the minimum standards required by law, and are also going the extra step to achieve excellence in animal care and use.” The University’s Animal Care and Use Program has been fully accredited by AAALAC International since the 1980’s with site visits occurring every three years.
Authority
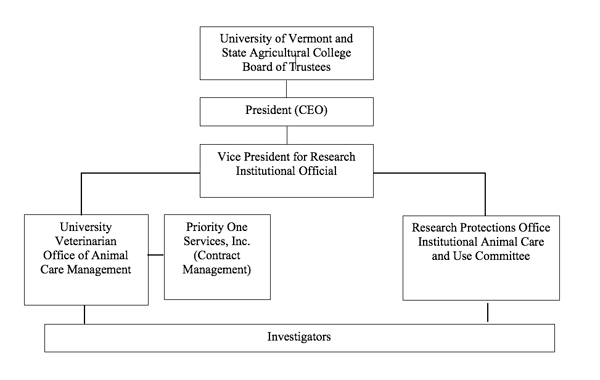
The Vice President for Research Administration (Institutional Official) (IO), as delegated by the President of the University, is responsible for the assurance of compliance with the humane care and use of animals for research and teaching at the University.
The Office of Animal Care Management (OACM) is directed by the University Veterinarian, who provides oversight and clinical care for animals utilized in teaching and research activities. The University Veterinarian and the OACM are under the authority of the Vice President for Research.
The IACUC is established by the authority of the Vice President for Research to comply with the federal regulations governing all research in which vertebrate animals are used.
Scope
Animal welfare and IACUC policies apply to all faculty, staff, students, and visitors. IACUC policies apply to research and teaching activities that are:
- Sponsored by UVM
- Conducted by UVM researchers,
- Classes taught by UVM faculty, or
- Conducted using UVM property or facilities.
Mandated Functions
The following points are the federally mandated functions of the IACUC according to the Animal Welfare Act, Guide for the Care and Use of Agricultural Animals in Agricultural Research and Training and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
- Protocol Review: The IACUC is required to review and approve protocols, or changes in protocols, involving the care and use of animals in research, testing, or teaching activities
- Authorized to Suspend Activities: The IACUC is authorized to suspend an animal care and use activity that fails to comply with federal regulation or an IACUC approved protocol
- Animal Facility and Laboratory Inspection: At least every six months, the IACUC is required to inspect all UVM animal housing facilities and animal use area (laboratories), using specified standards as the basis for evaluation and reporting
- Animal Care and Use Program Review: At least once every six months, the IACUC is required to review UVMs program for the care and use of animals, using specified standards as the basis for evaluation and reporting
- Make Recommendations: The IACUC makes recommendations to the IO regarding any aspect of UVM’s animal facilities or program
- Review Concerns: The IACUC reviews concerns involving the care and use of animals at UVM
Resources
The institution will provide the IACUC with resources, office space, professional staff, and support staff sufficient to carry out their responsibilities efficiently and effectively and to serve as day-to-day liaison with appropriate University administrative offices, project investigators, other institutional safety and ethics boards, and various regulatory and funding agencies.
3.0 Committee Membership
Revised: 3/10/23
Committee Leadership and members serve at the discretion of the Institutional Official (IO). The IO has delegated signature authority to the Executive Director of Research Administration and Integrity for the appointment letters.
Chair
- Committee Chairs are appointed by the IO.
- A Committee Chair must be a University faculty member and must have prior service as a Committee Member.
- It is the responsibility of the Committee Chair to conduct Committee meetings in accordance with established federal regulations and University operating policies and procedures. These responsibilities, include but are not limited to the following:
- sign official Committee action documents and verification of approval forms
- keep abreast of relevant state and federal regulations;
- meet as needed with the IO to discuss Committee activities;
- keep abreast of procedures for maintenance of official protocol files and other administrative operations of the Committee;
- meet regularly with appropriate representatives of the RPO, IO, and the Office of Animal Care Management to coordinate the review of animal care and use throughout the University;
- recommend, in consultation with the IACUC Director, new Members to the IO;
- ensure that new Members are properly oriented to and educated about their duties and responsibilities
- initiate activities designed to keep the campus and community apprised of IACUC activities; and assist appropriate University administrators in the preparation of federal reports and assurances and meet with inspectors/site visitors as necessary;
No method for removal is delineated, as all Members are appointed and serve at the discretion of the IO.
- Appointment of the Chair is for renewable two-year terms.
Associate Chair
- The Associate Chair must be appointed from the regular voting membership
- Associate Chair will conduct the meetings of the IACUC in the absence of the Chair or if there is a conflict of interest by the Chair's participation
- Associate Chair will assist in the conduct of designated and administrative review of protocols
- Associate Chair will sign official Committee action documents and IACUC verification of approval forms and approve Committee actions as delegated by the Members or by designation of the Chair.
- The Associate Chair is appointed for renewable two-year terms.
Regular Members
Members will be of diverse professional and personal backgrounds and must demonstrate a genuine interest in and commitment to the purpose of the Committee. Specific Membership criteria must comply with all relevant federal and state regulations. Every effort will be made to fulfill principles which embrace cultural diversity.
- The IO appoints all Committee Members, after receiving recommendations from the IACUC Chair, and the IACUC Director.
- When vacancies occur, suggestions for Membership will be sought, after which formal recommendation(s) for new Member(s) will be made by the Committee Chair and the IACUC Director to the Institutional Official.
- The IO will in no case make a final appointment without prior consultation with the Committee Chair, and/or the IACUC Director.
- Committee appointments will usually be for renewable two-year terms, serving at the discretion of the IO.
- Consideration is given to achieve a balance between new and experienced Members when determining which appointments will be renewed.
- No specific attendance requirements are delineated - however it is required that Committee Members demonstrate a genuine interest and commitment to the purpose of the Committees. If a committee member is consistently absent from monthly meetings, a discussion with the IACUC member will take place to determine if interests have changed and whether the appointment should continue.
- No method for removal is delineated, as all Members are appointed and serve at the discretion of the IO.
Ex Officio Members
An ex officio member is defined as a member who serves by virtue of an office or position held. An ex officio member may be appointed by the IO as a voting member, a non-voting member, or an alternate member (see below).
- The University Veterinarian will serve ex officio as a regular member with full voting privileges.
- The RPO Director will serve as an ex officio member. This individual may serve as an alternate member if so appointed.
- Additional ex officio or alternate members may be appointed at the discretion of the IO.
Alternate Members
An alternate member is defined as a member who substitutes for a specific member or members with similar qualifications, experience or membership category. When an alternate member substitutes at a meeting, they appear as “substitutions” on the minutes. Alternate members may be appointed under the following conditions:
- Must be appointed by the IO and listed in IACUC rosters submitted with regulatory documents.
- Must be designated to serve as the alternate for a specific member or members who have the same attributes (e.g. scientific member can only substitute for another scientific member.)
- Alternates must receive same onboarding training as non-alternate members.
- Must receive all proposal materials in advance of the meeting for review if they will be voting during the meeting.
- Alternate members are advised to "vote their conscience" as opposed to representing the position of the regular member for whom they serve.
- If both the regular voting and alternate member both attend a meeting, only the regular voting member may vote. An alternate member may only be required to vote when necessary to achieve or maintain quorum.
Committee Composition
At a minimum the Committee will consist of not less than five regular voting Members, and at a minimum will include representation from each of the following:
- one Doctor of Veterinary Medicine, with training or experience in laboratory animal science and medicine, who has direct or delegated program responsibility for activities involving animals at the institution;
- one practicing scientist experienced in research involving animals;
- one Member whose primary concerns are in a nonscientific area (for example, ethicist, lawyer, member of clergy); and
- one individual who is not affiliated with the institution in any way other than as a Member of the IACUC, and is not a member of the immediate family of a person who is affiliated with the institution.
An individual who meets the requirements of more than one of the categories detailed above may fulfill more than one requirement, if the minimum number is met.
Consultation
The Committee may, at its discretion, obtain consultation from individuals with expertise in specialized areas to assist in the review of complex issues which require expertise beyond or in addition to that available on the IACUC. The IACUC shall consult with General Counsel and other University Officials, as indicated, to address issues pertaining to institutional policies, applicable law, and standards of conduct and practice. These individuals do not vote.
General Liability Insurance Coverage
Actions by the Members carried out as a function of their Committee appointments are included under the University's general liability insurance coverage.
Monetary Compensation
Regular membership on the IACUC will be without monetary compensation.
Conflict of Interest
If an IACUC Member has a conflicting interest in a protocol (including, but not limited to being a Principal Investigator (PI), a co-investigator, or a consultant on that protocol), that Member may only provide information as requested by the IACUC and will not be assigned to officially review nor vote on that protocol.
Code of Conduct for Members
This Code of Conduct is a set of behavioral expectations intended to assure that our Committee members uphold the highest level of integrity and ethical standards. Members must not discuss, disclose, or reproduce any protocol-related information, except as necessary to carry out responsibilities or as required by law. Members must limit their electronic access to that which is required to fulfill their Committee duties. Members must never access any research protocols to satisfy personal interest or curiosity. Any printed materials for review should be returned to the IACUC office, shredded after use or deleted from electronic devices.
Committee Member Training
The chair or the chair’s designee, meets with new members prior to attendance at their first meeting. The purpose of this meeting is to provide background and context to the committee’s responsibilities; to provide orientation to the committee’s operations and procedures, and to review the apprentice program. The committee's history, the Animal Welfare Act, the Public Health Service Policy, and the Guide are reviewed. The new member is provided with all the IACUC website addresses to access materials prior to their first committee meeting.
Additional training for IACUC members includes a four-step apprenticeship program. Each new member is “apprenticed” to a “veteran” committee member. The apprentice and veteran are matched and meet each other at the first IACUC meeting. The roles of apprentice and veteran are as follows:
Apprentice’s First Meeting At the new member’s first IACUC meeting, they simply observe the conduct of the meeting.
Apprentice’s Second Meeting The new member is assigned a “shadow” review and receives the same materials as a veteran reviewer. The new member does not need to present anything to the Committee but can compare their review with the veteran’s review as part of the learning process. The new member is welcome to comment and to ask questions during the meeting
Apprentice’s Third Meeting In this case, the new member is assigned a review and is paired with the veteran member. The new member consults with the experienced reviewer, the staff or the Chair if there are any questions or concerns during the review. The new member presents the protocol and his/her views. The veteran reviewer provides assistance if there are any concerns during the review presentation at the meeting.
Apprentice’s Fourth Meeting The new member performs his/her own review and presentation at the IACUC meeting as the primary or apprentice reviewer of the protocol. In this case, an experienced committee member will serve as the other reviewer.
From that point on, the new member will assume full committee responsibilities. However, staff, the chair and the entire committee function as a team to support every member. Prior experience on an IACUC may substitute for some or all of the mentorship program requirements, as determined by the IACUC Chair.
Documentation of Training Completion
Records of completion dates are maintained in RPO shared folder S:\irb\Committee Member Management\Rosters.
Continuing Education
Continuing education is accomplished by retaking the IACUC CITI Trainings at least once every three years, attendance at webinars, regional or national meetings and conferences. Additional education is provided as topics discussed during the monthly Committee meetings.
4.0 Operations of the IACUC
Convened Meetings
The Committees meet monthly if there are agenda items.
Meeting Notices
Meetings are noticed on the IACUC website in conformance with the Vermont Open Meetings Law (1 V.S.A. Section 310.) The agenda, including the time and location of the meeting, and pre-meeting materials for protocols to be reviewed, are distributed in advance to all Members.
Official minutes
Minutes will be kept of all convened meetings.
Conducting Required Reviews
Initial and continuing IACUC reviews and approvals of a protocols will occur in compliance with the Public Health and Safety (PHS), the United States Department of Agriculture (USDA) requirements and other regulators as required. Reviews will be preceded by IACUC receipt of appropriate electronic SMART forms in the UVMClick-IACUC system from the investigator. Reviewer forms and internal checklists are utilized as a guide to ensure that these criteria have been met.
Review Decisions and Process for Appeal
Review decisions will be forwarded to the researcher in writing. Review decisions may not be overridden. However, the IO shall have the final authority to disapprove, restrict or terminate a study which has received IACUC approval. There is no process delineated for appeal of Committee decisions. However, there is no prohibition for resubmission of specific requests or protocols for additional review by the Committee.
Protocols Requiring Additional Review
Determination of which studies require review more often than annually is done at the time of protocol review, on a case by case basis, depending upon protocol specific factors, including, but not limited to, the level of risk.
Modification to a Protocol
The IACUC requires that changes in approved research be reviewed prior to initiation. Changes implemented prior to Committee approval is considered noncompliance.
Concerns
The IACUC is required to report significant problems and violations of the regulations.
Removal from Consideration
RPO staff will have the authority to remove from further Committee consideration, a study which has obtained initial approval, when the PI fails to respond to ongoing clarifications or training requests. The PI will be required to close the study in UVMClick-IACUC.
Documentation
The IACUC, through the administrative staff, is responsible for reporting findings, actions as well as requesting clarifications to the investigators in writing, and to the appropriate offices within the institutions’ administration through reports and meeting minutes to institutional officials through their representatives on the Committee, and to sponsors of research, if so required.
Authority to Sign IACUC Documents
a. Results of Reviews, Actions and Decisions whether Full or Expedited
Depending upon the nature of the required conditions, the IACUC designates any of the following individuals or groups of individuals to determine that the conditions of approval have been satisfied:
- The IACUC chair;
- Another member;
- An IACUC Analyst/Member or;
- Other qualified IACUC administrative staff person, who need not be an IACUC member.
Individuals designated by the IACUC must have appropriate expertise or qualifications. For some conditions, the review of response materials from investigators will require scientific or other technical expertise. In such cases, the IACUC Chair will review the responsive materials or designate another individual who has the appropriate expertise.
The following are some examples of conditions in which IACUC Analysts/Members have been designated to review and approve response materials.
- Correction of minor grammatical and typographical errors in the protocol;
- Requirement to revise the protocol in a specific manner as dictated by the Committee.
b. Administrative Review and Approval
Administrative items are reviewed and approved by IACUC Analysts/Members or appropriate IACUC staff. IACUC Analysts/Members may consult with the Committee chair prior to approval. Below are examples of administrative items.
- Changes to Key Personnel
c. Routine Internal Correspondence
Any action, letter, memo or e-mail between the Committee or IACUC staff and the faculty or staff of the University that provides information concerning the review of research protocols by the Committee or IACUC staff and which do not imply or appear to imply approval of this activity may be signed by the staff member.
d. Decisions Made by the Chair
Any letters, memos or email sent representing the decision or opinions of the Chair or Vice Chair of the IACUC, as long as such correspondence does not imply review and approval, may be signed by IACUC staff if so designated by the IACUC.
Electronic Reviews
All reviews, initial, continuing reviews and modifications are completed electronically by the IACUC members as assigned. Members receive an email notice that a review is pending. Members are required to authenticate into the electronic system using their UVM NetID and password prior to completing their review. The system validates the member’s authentication credentials based upon the member’s role in the system and determines available actions for each person. The electronic review is stamped within the system with the name of the individual carrying out the review activity (electronic signature), and the time and date that the electronic signature was applied to the review.
Members can only access records for the purposes of fulfilling their committee review duties.
Voting Requirements
- A majority of the total number of regular voting members will constitute a quorum. The number in attendance, including alternate members who are alternating for regular members, must be one more than half the total number of regular voting members. If less than a majority of the total number of regular voting Members is present, if an ex officio nonvoting member(s) is available, that individual may be included to constitute a quorum. If a quorum is lost at any time during the meeting, the meeting shall be adjourned and no further action taken until a quorum is attained.
- Official Committee action on protocols involving vertebrate animals will be by formal vote at convened meetings of a quorum of Committee Members, utilizing approved Review Action Categories.
- All meetings will be conducted using Robert’s Rules of Order as guidance, with deviations made as deemed appropriate by the Chair.
- Selection of specific protocols for review by members is determined by the Committee's administrative staff and/or Chair. If an IACUC member has a conflicting interest in a protocol (including, but not limited to being a PI or a co-investigator) that member may only provide information as requested by the IACUC and will not be assigned to officially review nor vote on that protocol.
- No Member may vote on any matter in which that Member has a conflicting interest (e.g., the Member is personally involved in the project, has performed internal review for scientific merit, or is a consultant for the project).
- IACUC members may participate in a convened meeting of the IACUC via telephone or video conferencing. Those members have access to the research protocol materials in advance of the meeting within the UVMClick-IACUC.
IACUC Record Requirements
The IACUC keeps all records in accordance with all pertinent regulations. All records are accessible for inspection and copying by authorized OLAW or other PHS representatives at reasonable times and in a reasonable manner. This record keeping includes:
Membership rosters - The institution is required to maintain a current list of IACUC members identified by name; earned degrees; representative capacity; indications of experience such as board certifications or licenses sufficient to describe each member's chief anticipated contributions to IACUC deliberations; and any employment or other relationship between each member and the institution (e.g., full-time employee, part-time employee, member of governing panel or board, stockholder, paid or unpaid consultant). UVM rosters indicate regular voting versus alternate members, as well as alternate replacement assignments. Rosters are updated each time there is a change in the Committee membership. Copies of curriculum vitae are obtained and kept on file for all primary and alternate members.
Written procedures and guidelines including, but not limited to, the Research Manual and all website content.
Animal Welfare Assurance and any modifications thereto, as approved by PHS
Minutes of meetings document
- attendance of members, consultants, and guests
- discussion of controverted issues,
- actions taken,
- basis for requiring changes in research or disapproving research,
- a record of voting (for, opposed, abstaining, recusal),
- members arriving and leaving the meeting once the meeting is called to order,
- names of members who recuse themselves because of a conflict of interest along with the fact that a conflict is the reason for the recusal,
- which alternate member is replacing a primary member,
- a record and vote of members who participated in the convened meeting via phone or video conferencing.
Approved minutes will be signed by the Chair or designee, scanned, and maintained as a PDF in a shared electronic file.
A report of business conducted by the expedited review process is available in the UVMClick-IRB system.
Semiannual IACUC Inspection reports and recommendations (including minority views) as forwarded to the IO, the Vice President for Research.
Accrediting body determinations
Protocols, continuing reviews, amendments are kept electronically in the UVMClick-IACUC system.
Problems or adverse events and IACUC reviews, in accordance with IACUC policy, specific federal regulations and the Assurance;
Communications to and from the IACUC are maintained electronically in the protocol record.
4.1 Coordination with Other Compliance Committees
The IACUC is not the only entity responsible for oversight of animals used in research. The Office of Animal Care Management, Risk Management, and UVM biostatisticians are examples of entities or individuals across UVM that play roles in protecting animal use research. Unique requirements may be in place if your research activity is performed with the support of one of these units.
Coordination includes sharing of protocol data. The UVMClick system has more than one mechanism to share protocol information with those stakeholders. An individual may be provided with the Global Viewer role, which means that they can view every protocol in the system but not edit it. The Global Viewer role is only assigned to those individuals, as chosen by their supervisor, for specific tasks related to their position requirements and requires prior approval by the RPO Director.
The more common way that we allow access to protocol information is through the Ancillary Review mechanism. This limits an individual’s access to just those protocols where information is required for the Committee/Division to assess feasibility, coordinate and develop process with the investigator, ensure contracts are complete, etc.
Assignment of ancillary reviews allows those Committees/Divisions access to your protocol materials. They will in turn work directly with you regarding any clarifications or items that require resolution. Once the submission is acceptable to them, you will be required to resubmit any revised documents back through the IACUC system so that the IACUC reviewers have the most current version to conduct their review.
Each Committee/Division has identified different stopping rules for the ancillary review. Completion of an ancillary review may have no direct impact and is simply an FYI for the Committee, or it may be a condition of IACUC approval release or IACUC review may be held until the ancillary review is complete. Examples of Committees/Divisions ancillary reviews that may be required during the protocol and/or modification review process are consultants from Risk Management and Safety, biostatisticians, faculty sponsors, or Animal Care staff.
4.2 Projects Utilizing Biohazards in Animals
All projects proposing to utilize recombinant DNA or infectious agents require review and approval from the Institutional Biosafety Committee (IBC). PIs may submit an IBC and an IACUC protocol simultaneously; however, release of the IACUC approval is pended until the IBC protocol has been approved.
The IBC is charged with reviewing all research projects and activities involving recombinant DNA (as outlined in the “Guidelines for Research Involving Recombinant DNA Molecules”) to assure that specific practices for constructing and handling (i) recombinant DNA molecules and (ii) organisms and viruses containing recombinant DNA molecules are followed. The IBC is also charged with responsibility for reviewing the use of infectious agents in research at UVM. A representative of the Office of Environmental Safety serves on the IBC, as does the Director of the Department of Risk Management, the University Veterinarian, the Radiation Safety Director, and faculty of the College of Medicine with particular expertise in infectious agents.
For more information go to https://www.uvm.edu/rpo/biosafety-oversight and www.uvm.edu/riskmanagement/radiation-safety.
4.3 Criteria for Approval
All proposed activities are reviewed to ensure that the following federal requirements for granting IACUC approval are met:
Activities -- All activities involving animals must be in accordance with USDA Regulations/PHS Policy.
Animal numbers and group sizes – The IACUC requires that the experimental design be described and estimated animal numbers justified either with a power calculation or a reference to previously-published work of comparable scientific design. A biostatistician serves on the Committee and has the specific charge of evaluating the numbers justification section. A biostatistics tutorial providing guidance for the investigators was launched in the spring of 2013.
Harm/benefit analysis – All protocols which entail more than momentary pain/distress (reference USDA category D or E) are reviewed in a convened meeting of the IACUC. During the discussion, the IACUC notes the responses of the investigator to the rationale for utilizing animals and the potential significance of the work. For protocols which entail more than momentary pain/distress without provision of analgesia, a specific scientific justification for withholding analgesia is required. If the Committee has further questions regarding the harm/benefit analysis, the investigator may be required to provide further clarification prior to approval of the protocol.
Search for alternatives – In the case of any protocol which entails more than momentary pain or distress to the animals, the investigator must perform a literature review seeking alternatives which could achieve the same scientific objectives with a lesser degree of pain/distress. The investigator must describe in a narrative what leads him/her to the conclusion that there are no alternatives to the proposed procedures.
Rationale and Methods -- All proposals must include:
- Identification of the species and the approximate number of animals to be used;
- A rationale for involving animals and for the appropriateness of the species and numbers of animals to be used;
- A complete description of the proposed use of the animals;
- A description of procedures designed to assure that discomfort and pain to animals will be limited to that which is unavoidable for the conduct of scientifically valuable research, including provision for the use of analgesic, anesthetic, and tranquilizing drugs where indicated and appropriate to minimize discomfort and pain to animals; and
- A description of any euthanasia method to be used.
Note: Some investigators have requested to use video surveillance or videotaping as part of their protocol. Video surveillance or videotaping cannot be used as a substitute for in-person protocol monitoring activities and there are strict criteria about controlling video access, especially if it is being viewed externally. See 15.36 Policy on Photography or Video Monitoring of Animals. Contact the RPO for advice.
Duplication -- Assurance that activities do not unnecessarily duplicate previous efforts must be provided.
Surgery -- Requirements for sterile surgery and pre/postoperative care must be met. An animal may not be used for several major operative procedures from which it will recover, without meeting specified conditions.
Euthanasia--The euthanasia method must be consistent with the recommendations of the current AVMA Panel on Euthanasia (2022 edition or later).
Housing/Health -- Animal living conditions must be consistent with standards of housing, feeding and care directed by veterinarian or scientist with appropriate expertise. Medical care must be provided by qualified veterinarian.
Qualifications --Personnel must be appropriately trained and qualified. Completion of all the University of Vermont's Office of Animal Care Management training program is required for all individuals working with animals or identified on a protocol.
Deviation from Requirements --Must be justified for scientific reasons, in writing.
The Committee’s review process always includes a check for compliance with all applicable IACUC or institutional policies and procedures.
Congruency -- The IACUC protocol must align with the grant application that will support the animal studies. Differences between the IACUC protocol and the grant application must be addressed and reconciled.
4.4 IACUC Review Determinations
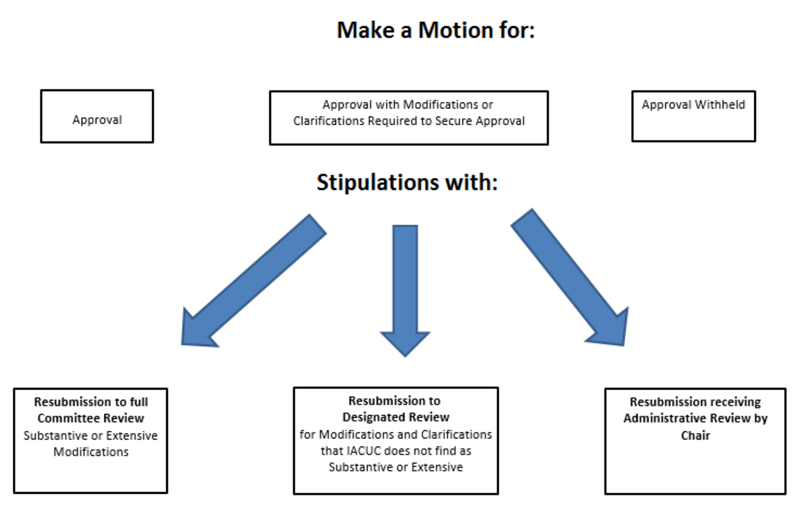
Written Communication of IACUC Decisions
Decisions made by the IACUC will be communicated to the PI (or designee if provided) through a memorandum outlining the approval status and/or concerns, questions and/or comments of the IACUC. This correspondence will be forwarded via the UVMClick system to the PI.
The IACUC notifies investigators and the institution of its decisions regarding protocol review through written memoranda and the minutes of IACUC meetings which are transmitted electronically. The decision to withhold approval is communicated to the investigator along with the reasons for withholding approval. There is no appeal process for the IACUC’s decision to withhold approval; however, an investigator may address the IACUC’s concerns and submit for re-consideration.
The IACUC Chair or other member will convey one of the following four decisions in writing to the investigator promptly after the meeting:
Approval
The PI may begin the research study upon receipt of the Verification of Approval.
Modifications Required for Initial or Continuing Approval
This decision is determined when the protocol is recommended for approval by the IACUC pending the investigator’s response to IACUC-directed stipulations/questions and/or revisions. The PI must provide a memorandum responding to the IACUC’s recommendations and make any changes to the IACUC protocol SMART form as applicable.
Depending upon the issues that have been raised, the review of the response may occur through a Designated or Full process.
Withhold Approval
Questions regarding the scientific merit and use of animals are of such significance that the committee finds approval of the study to be unwarranted. The authority of the IACUC to withhold approval of a study may not be overridden.
NOTE: The IACUC has a 30, 60, 90 day reminder system for all pending protocol items. The investigator is reminded that the IACUC has requested something from them in regards to a protocol and is awaiting his/her response. At the 120 day mark the protocol is withdrawn from the Committee’s consideration. This helps to ensure that changes to protocols are handled in a timely fashion.
4.5 Public Records and Open Meeting Requirements
Federal Freedom of Information Act (FOIA)
FIOA is a federal law that generally provides that any person has a right, enforceable in court, to obtain access to federal agency records. FOIA applies only to federal agencies. Each state has its own public access laws that are consulted for requests for access to state and local records.
Vermont Public Records Act
State open records laws are state statutes that govern access to records in the possession of state and local governments and other state public bodies, such as public universities.
The University of Vermont is a public body subject to the Vermont Public Records Act (1 V.S.A. §316)(a). Under this statute, any person may inspect or copy any public record of a public agency. The definitions of public agency; public records and documents are included in 1 V.S.A. §317.
Vermont State Exemptions to FOIA
Vermont statute exempts research data and protocols under §317(c)(23). Because these records are exempt from public disclosure by the State, the FOIA cannot be employed to inspect or copy records. Researchers should forward any request for inspection or copies of research records to the Vice President for Executive Operations according to the UVM Records and Document Request policy (PDF).
Vermont Open Meetings Law
The University of Vermont is a public body subject to the Vermont Open Meetings law §312. All meetings of a public body are declared to be open to the public at all times, except as provided in §313.
Executive Session Exemption under the Vermont Open Meeting Law
The Committee may only move into Executive Session to consider research protocols as exempted under §317(c)(23).
The Committees’ convened meetings begin in Open Session and move into Executive Session only after an affirmative vote of two-thirds of its present members. Such vote is taken in the course of an open meeting and the result of the vote recorded in the minutes. A motion to go into executive session must indicate the nature of the business of the executive session, and no other matter may be considered in the executive session. No formal or binding action will be taken in executive session. Once protocol discussions are complete, the Committee will vote to move back into Open Session to collect a formal vote on the research protocols that were discussed.
4.6 Conducting IACUC Business in the Event of a Public Health Emergency or other Significant Emergency
Regardless of external events, the Office of Laboratory Animal Welfare at NIH (OLAW) expects each institution’s IACUC to continue to conduct business according to requirements found in the PHS Policy, the Animal Welfare Act and Regulations, and the Guide.
IACUC Meetings
Regulations state that the IACUC must have at least 2 meetings a year, 6 months apart. A convened meeting with a quorum present must conduct the following: 1) suspension of a protocol 2) full committee review of protocols. While optimal, there is no requirement to conduct the convened meeting in person. Use of teleconferencing or audio/video conferencing is permissible. If quorum cannot be achieved, convened meetings will be postponed until enough members can be present.
Security of IACUC remote meetings will be assured by using only University-approved videoconferencing software logging in only with UVM credentials. Guest presence will be controlled by the meeting owner which, is typically a RPO staff person. IACUC videoconference meetings will not be recorded. Quorum of members will assured by a count of those in attendance prior to opening the meeting and a second time after moving into open session prior to the protocol vote.
While optimal, there is no requirement to conduct the semiannual review of programs at a convened meeting with a quorum present. We will assemble as many members as possible (minimum of two) to conduct the semiannual review of UVM’s program.
Minutes of meetings will be captured following current methods for in-person meetings. The manner of engagement of each member will be noted (e.g. in-person, telephone, video.) Votes to go in or out of sessions, as well as to vote for specific protocols, will occur by the Chair asking for members who approve an order of business by asking “All approved say aye”, “All opposed say nay”, “All abstaining say aye”. Members participating through video conferencing can also use the chat feature to add comments to the discussion. Members with conflicts will sign out of the meeting during the protocol discussion and IACUC staff will invite them back into the meeting when the conflicting protocol discussion is complete. Meeting guests will be invited during discussion of their protocol and signed out once that discussion is completed.
Approve/accept semiannual report to the Institutional Official by convened meeting (e.g. in-person, telephone, video). A majority of the members must approve report and any minority votes will be documented.
Protocol Review
The only federal requirement for Full Committee Review (FCR) is when an IACUC member specifically requests full committee review of a given protocol. Current IACUC policy requires the following protocols to be reviewed by the full committee:
- Procedures that may cause the animals pain or distress (i.e. survival surgery, Non-survival surgery, tumor burden studies, transplantable tumors, death as an endpoint, drug studies, prolonged restraint).
- Modifications of approved protocols to include procedures that have the potential to cause the animals pain or distress.
These protocol submissions must be reviewed in a convened meeting with a quorum present. Convened meetings can proceed as described above.
During an emergency, the Committee allows for Designated Member Review (DMR) of all protocols. All members will be notified ‘prior to the review’ as required. The Associate Chair may appoint DMR reviewers if the Chair is unavailable for an extended period. If both the Chair and Associate Chair are unavailable for an extended period of time, the IACUC Analyst/Assistant Director is authorized to appoint reviewers.
Protocols not requiring FCR may continue to be reviewed through DMR.
Every protocol involving USDA regulated species will continued to be reviewed annually through DMR.
Every protocol for a PHS funded activity will continue to undergo a complete review every three years either by FCR or DMR as allowed by the this procedure.
Protocol review will be documented through the UVMClick-IACUC electronic protocol submission software.
Semiannual Inspection of Facilities and Program Review
Delay semiannual inspections up to 30 days. Train veterinary technicians or other appropriate staff members in the animal care and use program to conduct facility inspections properly and have them assist with inspections as agents of the IACUC.
If personnel are not available, consider inspecting agricultural facilities at least annually as opposed to semi-annually.
If inspections need to be postponed as a result of the emergency, staff will document the date when the emergency began, which inspections were affected, whether a waiver was obtained from OLAW (USDA waiver is not necessary), when normal university operations resumed, when the inspections were reinstated and documentation the 6-month cycle was resumed.
Program evaluations will be conducted via in-person meeting, via teleconference or video on schedule. Minutes will be taken of that review including any minority opinions.
Training
If personnel refresher training expires during the emergency, personnel are allowed to continue their work with animals as long as they have received previous training and have demonstrated proficiency. There will be no consequences; however, once working conditions have been restored, personnel must complete refresher training in accordance the IACUC policy.
Pause on Animal Research Activities
If animal activities are required to be placed on pause secondary to institution-wide policy to address a public health situation, the IACUC does not require notification in the pause of work. A plan for continued care of the animals must be in place and protocols must remain in compliance with their expiration dates during a pause in work.
Once working conditions are restored, necessary changes to the protocol as a result of the public health situation, must be submitted for prior IACUC review and approval.
4.7 Adverse Event Assessment and Reporting Plan
New Policy 03/10/23
Purpose
The use of animals in research or teaching may occasionally result in an adverse event or unexpected outcome which may compromise animal welfare by causing or increasing the risk of pain/distress, morbidity, or death of the animal subject. To meet the obligations of our Assurance to the Office of Laboratory Animal Welfare (OLAW), our registration with the United States Department of Agriculture (USDA), and our AAALAC International continuing accreditation requirements, the University of Vermont must have a mechanism for reporting and evaluating these events in a timely manner. The Institutional Animal Care and Use Committee (IACUC), in its oversight of animal care and use, must ensure there is a clear path to report these events to provide the highest quality animal care. The IACUC Policy and Procedure (P&P) Subcommittee is tasked with review of these cases.
The scope of this policy applies to any unanticipated adverse events occurring in biomedical, agricultural and wildlife research animals.
Definition
Unexpected Adverse Events: An unexpected adverse event is an occurrence of an unforeseen event that negatively affects the welfare of research animal(s); that is, an event that involves pain, distress, and/or death of the animal. These are events that are not identified as a possible risk or outcome in the IACUC approved protocol.
Expected Events There may be levels of morbidity and mortality in virtually any animal-related activity, including those associated with the care and use of animals in research, testing, and teaching that are not the result of violations of either the PHS Policy or the Guide.
Animal Welfare Concerns: Concerns or deficiencies in the care and/or treatment of animals or any activities related to animal care that appear improper or inhumane.
Non-Compliance: Non-compliance occurs when an approved IACUC protocol, policies, procedures, or decisions are not followed.
Procedures
Animal welfare concerns as defined above must be reported to the IACUC for review following the IACUC procedures under Section 6.4 Animal Welfare Concerns.
Noncompliance as defined above must be reported to the IACUC for review following the IACUC procedures under Section 12.0 Noncompliance Policy.
When in doubt, contact the IACUC office or the University Veterinarian.
Examples of occurrences that must be reported as an unexpected adverse event:
- Unexpected clinical signs, either related or unrelated to a protocol procedure
- Surgical complications, which may include recurring unexpected anesthetic deaths
- Animal morbidity or mortality more than that described in the approved animal use application, including endpoints prompting euthanasia that were cited in the protocol and found to be inadequately predictive resulting in unexpected animal deaths
- Unexpected circumstances that lead to animals being subjected to obvious harm or distress that is not justified and approved by the IACUC such as facility or weather-associated events. Examples include HVAC or power failure, flooding, fire, housing malfunctions.
Examples of occurrences that are not required to be reported as they are not unexpected events:
- Death or morbidity of animals that has been described and approved in the animal use protocol (e.g. increased mortality rates due to described phenotypic characteristics of transgenic lines, post-operative complications).
- Mortality increases as a function of age in all animals, the death of aged animals due to natural causes is not considered an unexpected adverse event
- Injury or illness unrelated to approved research procedures – for example, dermatitis or species-specific behavior (among rodents could include fight wounds, barbering, and neonate cannibalization).
- Note: If these situations are noted to be increasing or in high numbers, it may be an indication of an issue and should be addressed with the veterinary staff.
- Death or failures of neonates to thrive when husbandry and veterinary medical oversight of dams and litters was appropriate
- Animal death or illness from spontaneous disease when appropriate quarantine, preventive medical, surveillance, diagnostic, and therapeutic procedures were in place and followed
- Animal death or injuries related to manipulations that fall within parameters described in the IACUC-approved protocol
Process for Review of Unexpected Adverse Events
All unexpected adverse events must be reported to the IACUC through a “Concern” submission from the PI or their designee. The PI must contact the IACUC staff for assistance in submitted this report. It is possible the IACUC may become aware of an unexpected adverse event through other means such as the University Veterinarian or Animal Care Staff, an independent reporter, or an external source such as AAALAC or USDA site visitor. In these instances, the IACUC staff will enter the concern into the system. The IACUC Monitoring and Compliance Specialist makes an initial decision as to whether the concern being reported meets any of the above definitions. The Specialist may make an inquiry to the researcher for additional information, or they may consult with a P&P Member if scientific expertise is necessary to assist in making the determination. There are three outcomes of this initial review. If the concern:
- does not meet any of the definitions noted above, or it is an expected event, the concern will be acknowledged, and a memorandum will be sent to the PI explaining the outcome of the review and whether any further actions are necessary. No reporting is required in these instances.
- indicates potential as a serious adverse event or valid animal welfare concern, it is placed on the next available P&P agenda as New Business. The concern will be assigned to a P&P Member (primary) reviewer. All P&P Members are provided access to the concern, which includes a description of the issue, proposed corrective actions, as well as access to the currently approved protocol. The primary reviewer will summarize the issue, proposed corrective action and his/her preliminary recommendation as to whether the concern is reportable and whether the proposed corrective actions are appropriate.
- is determined to be a result of noncompliance, the matter will be managed through the IACUC Noncompliance Policy procedures.
Reporting to Regulators
If the P&P has determined the concern is not reportable, the Subcommittee will close out the issue with a memo to the PI. If further actions are felt necessary to mitigate recurrence, that will be included in the correspondence with the PI. The Specialist will ensure whatever actions are required are completed by the PI. This activity will be documented in the P&P Subcommittee minutes and is available to the Full Committees as applicable.
If the P&P has determined the concern requires further reporting, a recommendation and summary report of the concern will be written and forwarded to the Full IACUC Committee for review and vote. If the Full IACUC concurs with the Subcommittee’s recommendation, the summary report will be finalized and forwarded to federal regulators, AAALAC, Institutional Officials, UVM Compliance and Legal Counsel and applicable sponsors. Concerns that are referred to the Full Committee are included in the convened meeting minutes.
5.0 Types of Committee Review
The IACUC will review and have authority to approve, require modifications in (to secure approval), or disapprove all research and teaching activities involving animals. These review categories are employed for new projects, amendments, and continuing review.
The process for reviewing and approving protocols using animals begins with the completion of a protocol SMART form in UVMClick. Information required for the protocol is consistent with requirements detailed in the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the Animal Welfare Act and Regulations.
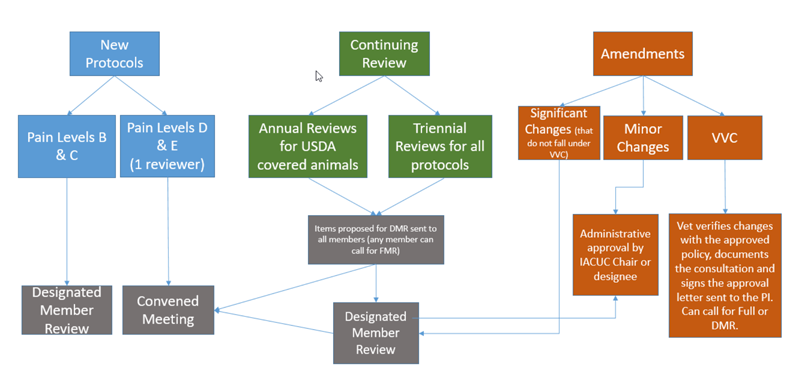
There are four types of Committee review: FCR, DMR, Administrative Review, and Veterinarian Verification and Consultation. Determination of the type of review is based upon the expected animal discomfort (reference the USDA pain levels) and types of procedures. This determination is made by the IACUC chair or the chair’s designee in consultation with IACUC staff or veterinarian as appropriate. The IACUC review process is presented schematically below:
5.1 Preliminary Reviews
In all cases, there are preliminary reviews as described below that require completion prior to review by the Committee.
Scientific Merit Review
IACUC review efforts focus on the appropriateness of animal numbers, procedures and adequacy of investigator skills. Scientific peer review normally is left to outside funding agencies. In lieu of outside review by a funding agency (e.g., internal funding, pilot studies or new investigator start-up), IACUC requires the department of record to certify that funds are awarded on the basis of scientific merit and periodic evaluation. This internal review can be done one of two ways; 1) through an internal review committee or 2) through independent scientific review which requires the sign-off of two knowledgeable faculty members. In both cases, the Scientific Research Plan and References must be examined. Please note that if a Faculty Sponsor is listed on the protocol, the Faculty Sponsor should not be the one conducting the scientific merit review.
For those protocols that require an internal review committee, an ancillary review will be assigned to merit reviewer(s) as indicated in the protocol submission and a response is required prior to protocol review by the IACUC.
Veterinary Review
Prior to any type of Committee review, protocol materials must be submitted into the UVMClick system where they will be forwarded to the veterinarian for review. A consultation with the University Veterinarian is required for all new projects requiring animals, including amendments to an existing protocol to add a new project. The USDA pain level is generally determined in this interchange between the investigator and the veterinarian. New projects submitted through UVMClick will be forwarded to the Veterinarian for the veterinary review. The completed veterinarian review will be forwarded electronically through the system.
IACUC Analyst Pre-Review
The IACUC must receive sufficient information regarding proposed activities to make the determinations as required by regulations. After veterinary consultation is complete, the IACUC Analyst will conduct a pre-review of submitted materials. A new protocol or amendment will not be added to an agenda until both of these reviews are complete. A specific regulatory checklist is followed to determine if the submission is ready for review. If the submission is incomplete or lacks information necessary to conduct a review, it will not be reviewed until the information is provided. Once determined to be satisfactory the review will continue.
5.2 Full Committee Review (FCR) (convened meeting)
The IACUC employs the convened meeting review process for protocols designated USDA Pain level “D” or “E”. Review may result in approval, a requirement for modifications (to secure approval), or withholding of approval. FCR must occur during a convened meeting of a quorum of the IACUC members, and with a formal vote.
Notification to Research Community
Committee meetings are noticed on the Committee website. Submissions are added to an Agenda after the Merit review (if applicable), Veterinary Review, and Analyst pre-review are complete.
Notification to the Committee
Distribution of the Agenda to all members occurs approximately 2 weeks in advance of the convened meeting. All new projects, concerns and other business requiring full committee action are placed on the Agenda for discussion.
Access to Protocol Materials
Committee members have access to all of the protocol materials through the electronic submission and review system.
Review Assignments
At least one reviewer is assigned by the Chair. More reviewers may be assigned at the Chair’s discretion. The reviewer is always a scientific representative of the Committee and is responsible for presenting a summary of the protocol at the meeting along with any concerns or points requiring clarification. The reviewer often will discuss their questions with the investigator prior to bringing the protocol before the full IACUC. If a secondary reviewer has been assigned, they will add any additional concerns, and any comments from the veterinarian are added. The protocol is then open for discussion by the full Committee, each member has had the opportunity to read and review the protocol prior to the meeting, and then a vote is taken. No member may participate in the IACUC review or approval of a research project in which the member has a conflicting interest (e.g., personally involved in the project) except to provide information requested by the IACUC. All members’ votes are recorded in the minutes of the meeting. An approval memo or a request for further clarification will be sent to the PI.
5.3 Designated Member Review (DMR)
Regulation
DMR may be utilized only after all members have been provided the opportunity to call for FCR or in instances when the quorum of members present at a full convened meeting have voted to review responses subsequent to the meeting using this method (see section below). If any member requests FCR then that method must be used.
Process
All IACUC members are sent an email notice of a pending designated review. The email includes a link directly to the protocol. Then the protocol goes into grace period.
All members have one business day to call for the item to be added to the Full Committee agenda. This time is referred to as the grace period. If there are no requests for full review, the IACUC Chairperson will appoint one or more qualified IACUC members to serve as the designated reviewer(s).
Designated Reviewer
The designated member reviewer may at any point call for a FCR. The IACUC Chair or Veterinarian also may call for a FCR at any point prior to approval. The Designated reviewer may request clarifications/stipulations prior to making a recommendation.
If a protocol is assigned to more than one designated reviewer, the reviewers must be unanimous in any decision. They must review identical versions of the protocol and if modifications are requested by any one of the reviewers then the other reviewer must be aware of and agree to the modifications. Protocol materials that meet the criteria for designated review are made available to the entire committee via Click.
Allowed Determinations under DMR
Designated review may result in approval, a requirement for modifications (to secure approval), or referral to the full committee for review. Designated review may not result in disapproval.
Protocol Transition from InfoEd to UVMClick-IACUC
The IACUC will utilize the DMR process to review and approve protocols as they are submitted into the UVMClick-IACUC system. Protocols will be transitioned (reviewed and approved by the IACUC) in accordance with PHS Policy Section IV.C.1-4 or USDA regulations 9 CFR 2.31(a)(5)(4).
5.4 Review of Protocol Modifications by Designated Member Review subsequent to Full Committee Review
The quorum of committee members present at the Full Committee meeting have the option to vote to return the protocol with modifications for FCR at a convened meeting or to employ the DMR as described previously. All IACUC members have agreed in writing that the quorum of members present at a convened meeting may decide by unanimous vote to use DMR subsequent to FCR when modification is needed to secure approval. However, any member of the IACUC may, at any time, request to see the revised protocol and/or request FCR of the protocol. This activity is in accordance with NOT-OD-09-035.
Written agreement from members has been documented and is on file on the RPO shared drive. New members will sign an agreement upon being added to the Committee.
5.5 Administrative Review
The chair alone (or his/her designee from the Committee) reviews and approves actions in this category. This category captures review of actions that do not require an actual Committee review (e.g., minor amendments, some continuing reviews, previously approved protocols that have been resubmitted unchanged or identical protocols submitted to different funding agencies, or protocols with no direct animal use).
5.6 Veterinarian Verification and Consultation (VVC)
Revised: 6/27/22
The VVC process may be used to make significant changes to animal activities that are part of an IACUC protocol that was previously reviewed and approved by FCR or DMR. The IACUC has granted authority to the University Veterinarian to handle specific changes administratively according to IACUC-approved policies. These changes include the following:
- Frequency, type, or number of procedures performed on an animal
- Changes to anesthetics, analgesics, sedatives, antibiotics, fluids, or experimental substances
- Euthanasia methods consistent with the AVMA Guidelines for the Euthanasia of Animals
VCC Process
Significant changes noted above must be submitted via the amendment process to an existing, IACUC-approved protocol for review. RPO staff will pre-review the submission and determine if it meets criteria for VVC. If it does, the analyst will then consult with the University Veterinarian to act in the capacity as a subject matter expert to verify that compliance with the IACUC-reviewed and -approved policy is appropriate for the animals in this circumstance. Consultation with the veterinarian will be conducted through UVMClick and the PI will receive written confirmation from the IACUC when the change has been reviewed.
DRUG FORMULARY FOR RESEARCH ANIMALS AT UVM
5.7 Sustainable Agriculture Research and Education (SARE) Grant Projects
Background
The Sustainable Agriculture Research and Education (SARE) program is a competitive grants program that funds research and education projects in every state and island protectorate. Funded by the United States Department of Agriculture's National Institute of Food and Agriculture, the program has four independently-run regions (North- Central, Northeast, South and West) hosted by land grant institutions and guided by regional Administrative Councils comprised of agricultural stakeholders. SARE Outreach provides communication and technical support at the national level.
The Northeast region is hosted by the University of Vermont (UVM). Northeast SARE offers six different types of grants, most of which are open to commercial farmers, educators, researchers, nonprofit organizations, public agencies and businesses. The prime award from USDA NIFA is awarded to UVM with the Director of Northeast SARE as the Principal Investigator. Grants under the prime award are awarded as subawards to institutions and as service agreements to individuals/private businesses, as is the case for farmers.
UVM has an obligation to assure that Northeast SARE’s grantees are aware of the need to comply with IACUC and IRB if their projects involve animals or human subjects. In most cases, grantees work with their home institutions or organizations to make that determination and develop plans as needed. However, small organizations and farmer grantees do not have this capacity.
Scope of Process
This process addresses awards made to individuals and organizations in Northeast SARE’s Farmer Grant, Partnership Grant, Research and Education, Research for Novel Approaches, and Professional Development programs. Whenever possible IACUC or IRB determinations will be made by an official review committee arranged by the awardee.
Projects Involving Animals
The University’s IACUC committee is not able to formally review projects from individuals who are not employees of UVM. However, if SARE receives a grant proposal that includes animals, the SARE program may request a UVM veterinary review of potential impacts on animal welfare. In that case SARE program staff will email the proposal and completed livestock care questionnaire to Dr. Ida Washington, University Veterinarian and Director, Office of Animal Care Management at University of Vermont, at Ida.Washington@uvm.edu. Dr. Washington will provide an independent review and a determination of whether or not the project meets appropriate animal welfare standards. This determination could include: that the project meets animal welfare standards; a request for clarification on aspects of a project; a request for specific changes to be made in project approach; or other determinations as appropriate.
SARE staff will work with the awardee to ensure Dr. Washington’s recommended changes are made prior to release of funds.
For awardees required to provide IACUC review outside of UVM, SARE staff will require that the IACUC review results be provided before research funds are released.
Projects Involving Human Subjects
The University’s IRB committee is not able to formally review projects from individuals who are not employees of UVM. The majority of proposals pose minimal risk in that they involve activities of normal daily living in which a reasonable person can choose to participate, or not. Projects of that nature would be exempt from IRB review. Recommendation of such exemption may be done by SARE program staff referencing materials found on UVM’s IRB website https://www.uvm.edu/rpo/determine-if-project-requires-irb-review, U.S. Department of Health & Human Service at https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts/index.html#c1 and, in the future, using a federally-developed self-determination tool.
SARE program staff may also contact UVM’s IRB staff for advice on exemption determinations in the event that a proposal selected for funding appears to pose more than minimal risk. Such projects then have two options:
1. After IRB staff offers guidance to SARE staff as to how the project can be changed to become minimal risk, SARE staff will require that the grantee modify the proposal accordingly before funding the project.
2. The grantee would be required to obtain an institutional collaborator with its own IRB board that can provide a determination and if needed, approve an IRB plan for the project.
Which of these two options is most appropriate will be determined on a case-by-case basis to determine the best course of action that supports grantees in implementing their projects safely and in accordance with human subjects protections.
For awardees required to provide IRB review outside of UVM, SARE staff will require that the IRB review results be provided before research funds are released.
6.0 Submission Requirements
The Click software requires SMART forms be completed for each type of submission. As noted in previous sections, there are preliminary reviews that must be completed prior to IACUC review. Once a protocol has been approved, the PI has the responsibility of informing the IACUC of changes in PI, key personnel, reporting concerns, submitting an annual and triennial continuing review, and closing the protocol when it is no longer needed.
6.1 Initial Protocol Submission
Protocol
The IACUC now accepts the model of the one protocol that has more than one sponsor. The decision on whether to combine protocols is left to the researcher. However, any protocols including USDA species or those projects that are Department of Defense (DOD) funded are required to be separate distinct protocols.
Deadlines
The deadline for submission of protocol materials for FCR is the first Monday of the month. Meetings are routinely held on the fourth Monday. The decision as to the type of review a proposal receives (full committee or designated) is based on the expected level of animal discomfort and types of procedures. Protocols are placed on the agenda on a first-come first-served basis.
6.2 Amendment to Approved Protocol
Revised 3/10/23
Modifications/Amendments to approved protocols must be documented appropriately, reviewed, and approved. An Amendment SMART form is completed requesting the modification including an explanation of the changes along with the rationale for the change. PI must complete an Amendment SMART form in UVMClick, revise the appropriate SMART form pages, and submit to the IACUC for review and approval. The IACUC Analyst, in consultation with the University Veterinarian, will determine if the modification is “minor” or “significant” utilizing the decision flow (below)
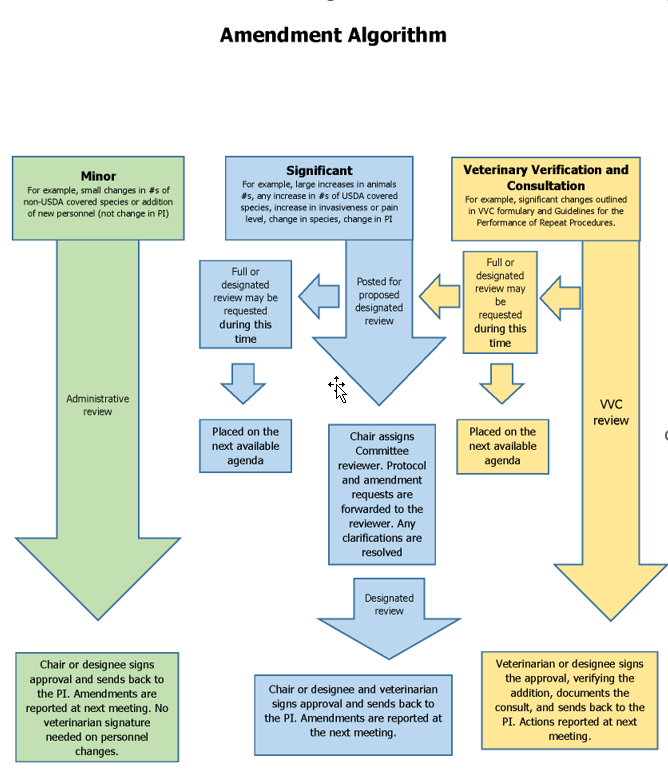
Minor modifications may entail such things as small changes in the numbers of non-USDA animal subjects or addition of new personnel (not change in PI). Other minor modifications may be approved administratively by the IACUC Chair and the University Veterinarian without FCR.
Personnel changes other than change in PI are administratively approved by IACUC staff only after confirmation that new personnel have completed the required training and occupational health form.
A significant modification may entail a large change in numbers of animals being used or requested, any requested increase in the numbers of USDA covered animals, an increase in invasiveness, a change in species, changing the route of drug administration, change in PI, or an increase in the USDA pain level. Addition of a newly funded project to an approved combined protocol is also considered a significant modification. Significant modifications require review either by FCR, DMR, or VVC.
Increases in animal numbers are considered significant changes to an approved protocol. Per PHS policy, the IACUC may review and approve this type of change administratively as long as the increase is not due to a change in study rationale or objective. Increases that may be handled administratively by the Chair or their designee must meet the following criteria;
- The increase is no more than 10% of the total number of animals listed on the study
- Study objectives and rationale for the use of animals remain unchanged by the increase
- Must be a non-USDA covered species protocol
If proposed increase in animal numbers is not eligible for administrative review and approval, it must be reviewed by FCR or DMR as determined by the University Veterinarian in consultation with the IACUC Analyst. The Chair may be consulted as needed.
6.3 Amendment to Add New Funded Project to an Approved Combined Protocol
This specific type of amendment will always be considered a significant modification. When a new grant is funded, if appropriate, an amendment to the existing protocol may be submitted to add any new animal work from the approved grant. The amendment SMART form should describe any new materials and any changes in research activities involving those animals.
These will be reviewed by either FCR or DMR as determined by the Chair.
6.4 Animal Welfare Concerns – Reporting
Revised 9/25/23
The IACUC is required by law to investigate reports of suspected animal abuse or mistreatment. If you observe or suspect animal abuse, mistreatment, or non-compliance with an approved protocol, you must report the incident. The IACUC will investigate and evaluate the concerns. All the information obtained from individual reporting, as well as information obtained during investigation, will be maintained confidentially.
All persons involved with the use of animals in research must know how to report concerns with animal care and use. There are no restrictions on who may report an alleged incident. No faculty employee, Committee members, or laboratory personnel shall be discriminated against or be subject to any reprisal for reporting violations or standards under the Act. The reporter may remain anonymous following the guidance below.
Submission and Review of Animal Concerns
All animal welfare concerns must be reported to the IACUC as soon as discovered by filing a Concern report in UVMClick. The Policies and Procedures Subcommittee of the IACUC will conduct an initial review. If necessary, clarifications will be requested to assist with review and determination. The Noncompliance Policy and Procedures document will be followed if the incident is suspected to be a result of noncompliance.
The IACUC will share information about the concern as applicable with the Office of Animal Care Management and the Institutional Official. Required reporting beyond the institution is discussed in the Noncompliance Policy and Procedures.
Definitions for the Concerns eForm
Deficiency: A deficiency is any deviation in policy, program, procedure, or facility condition from the standards described in the Guide, PHS Policy, or the AWARs which does not have a justified exception to those standards.
- Significant Deficiency
In the judgement of the IACUC, is or may be a threat to the health and safety of the animals.
This includes a significant deviation, defined as one that is or may be a threat to animal health, welfare, or safety (e.g., unapproved animal procedures that result in animal death, injury, or disease, failure to provide postoperative analgesics, failure to follow safety procedures, etc.) or is a substantial departure from IACUC policy (e.g., research or teaching involving animals occurring without IACUC approval).
- Minor Deficiency
In comparison to the significant deficiencies noted above, minor deficiencies refer to a problem for which an immediate solution is not necessary to protect life or prevent distress. Minor deficiencies in animal facilities include findings of moderate environmental fluctuations that are generally well tolerated, even if auxiliary equipment (i.e., heaters or chillers) may be needed to help minimize fluctuations, peeling or chipped paint, burnt-out light bulbs, missing floor drain covers, chipped floor surfaces, and similar problems.
This includes minor protocol deviations (e.g., failure to notify IACUC of new personnel, minor changes in drug administration or sampling frequency, etc.)
Protocol Deviation: is any departure from methods approved in an IACUC protocol.
Reporting Animal Concerns Anonymously
The University has an online compliance reporting system “Convercent” which can be accessed through UVM’s Compliance & Privacy Services. This system allows people to anonymously report an animal welfare incident and receive information about the follow-up without compromising their identity.
6.5 Continuing and Triennial Reviews
Revised 11/27/23
Protocols that are sponsored by certain funding agencies, require an annual continuing review. As part of the continuing review process, the Committee may require that the research be restricted, modified, reviewed more frequently or terminated/suspended. Alternatively, special precautions or Committee-imposed restrictions, or shortened review periods, may be modified if current data support such actions. Protocols covered by the Animal Welfare Act (USDA covered species) no longer require an annual continuing review. However, an annual post approval monitoring visit will be conducted.
Annual Continuing Review Process
For applicable protocols as mentioned above, UVMClick-IACUC will send an annual continuing review reminder to the PI and his/her delegated proxy three months before the approval of a protocol is due to expire. Additional reminders will be sent at two months and one month prior to expiration. The PI or proxy must log into the system and complete a continuing review SMART form and submit to the Committee for review.
The purpose of continuing review is to monitor: the status and progress of the protocol, and any animal use issues; that completed activities were conducted in accordance with the approved protocols; information about activities projected for the coming year, and; changes in key personnel and whether mandatory training is complete.
Triennial Review Process
All protocols, regardless of type and funding source, require a triennial review. The Triennial Review solicits the same information as the annual continuing review, but requires specific information about portions of the work which are completed or yet to be done. Prior to the end of the third year, UVMClick-IACUC will send a triennial review reminder to the PI and his/her delegated proxy three months before the approval of a protocol is due to expire. Additional reminders will be sent at two months and one month prior to expiration. The PI or proxy must log into the system and complete a triennial review SMART form and submit to the Committee for review. The Committee will conduct a complete review (i.e., de novo review) as required by PHS Policy. In addition, the Triennial Review requires a new literature review for alternatives to procedures which cause more than momentary pain or distress to the animals. The Triennial Review is completed by FCR or DMR.
Amendments at Time of Annual or Triennial Review
No new changes to the protocol are to be requested by the Investigator at the time of continuing review. Often, as part of the completion of the continuing review forms, it becomes apparent that an increase in animal numbers will need to be made. If Investigators need to make a change in the currently approved protocol, they must submit a separate amendment request through UVMClick. The same reviewer will be assigned the Continuing Review and any associated amendments whenever possible.
Approval
Approval will be provided by the Verification of Approval (VOA). The VOA indicates an expiration date which can only be extended through the Continuing Review process.
Expired Approvals
Extensions beyond the expiration date cannot be granted. In fact, the UVMClick system will automatically place the protocol in a lapsed state when the expiration date has passed.
Once expired, research activities are suspended and animals are moved to an institutional holding protocol, pending continued approval of the research by the Committee. Researchers found to be conducting research activities without a current IACUC approval are in noncompliance with the regulations.
6.6 Closure of a Project within a Combined Protocol versus Closure of the Combined Protocol
With the advent of the combined protocol, the IACUC needs to be notified and the combined protocol requires revision when a specific project has been completed. This is done through an amendment, not a closure, and will not affect the overall status of the combined protocol.
In the event that the entire protocol is closing, notification must be done by completing a Closure Request activity in UVMClick. The closure request will be reviewed by the IACUC and the researcher will be sent an official closure letter once the closure has been reviewed. The Office of Animal Care Management is notified by the IACUC of all closures. All animal use on a specified protocol is stopped and no further purchase of animals can be made under the specified protocol number.
6.7 Closure of Protocol and Amendments by Committee
New Policy 3/10/23
The IACUC may require that an approved protocol be closed in the following circumstances:
- If the work on a research protocol has not yet begun after a three-year period.
- If it is determined the principal investigator is no longer affiliated with UVM. In this instance, the Department Chair will be contacted to close the protocol or to assign a new PI.
- Closure may be required because of noncompliance. This would only occur after IACUC review and communication with the investigator. Termination of IACUC approval is reportable to the appropriate federal department or agency head and institutional officials.
In any of the situations described above, the IACUC office will notify the PI, as well as his/her department chair, of the study closure.
Removing from Committee Consideration (protocols/amendments not yet approved).
The IACUC may discard a protocol or amendment from continued consideration in the following circumstances:
New Full/Designated Member Review Protocols and New Amendments
- New protocols and amendments will be removed from consideration if the ancillary reviewer fails to respond within 30 days while in pre-review.
- New protocols and amendments will be removed from consideration (discarded) if the PI fails to respond to the Veterinarian’s initial request for revisions and/or clarifications within 30 days while in UVMClick clarification requested (vet consult) state, prior to Committee or Designated review.
- New protocols and amendments will be removed from consideration if the PI fails to respond to the IACUC’s initial request for revisions and/or clarifications within 30 days while in UVMClick clarification requested (pre-review) state, prior to Committee or Designated review.
- New protocols and amendments will be removed from consideration if the PI fails to respond to the IACUC’s request for revisions and/or clarifications within 30 days while in the modifications required state, after Committee or Designated review (in post-review).
- New protocols and amendments will be removed from consideration if the PI fails to respond to the IACUC’s requests for clarification within 6 months while in post review.
Notification of Committee Closure or Removal of Protocol
In any of the situations described above, the IACUC office will notify the PI, as well as their department chair, prior to study discard or closure.
Contact your Regulatory Analyst if sponsors, individuals, or processes outside of your control do not allow adherence to the response timeline criteria. Exceptions to response timeline criteria may be allowed on a case-by-case basis.
What If I Need to Reopen a Closed Protocol?
If an investigator needs to reopen a protocol after it has been formally closed with the IACUC, the investigator would be required to submit a new protocol for review and approval.
6.8. Closing a Protocol by the PI
New Policy 3/10/23
Investigators have the responsibility to formally close a study with current IACUC approval once it is completed. This informs the IACUC on the conduct and outcomes of the study, including any risks or problems that may have arisen since the last study renewal or approval.
Criteria for Closing a Protocol
Do not close-out a study if any of the following conditions apply:
- There are animals on this protocol in the animal facility(ies)
*note: The IBC and CSC must be notified of changes in IACUC protocol status if the protocol uses agents or controlled substances covered by an IBC or CSC registration. Please contact RPO with questions. In addition, other offices as applicable, such as the Radiation Safety Office, must be contacted to discuss closure requirements.
Notification of Closure to the IACUC
Notification must be done by completing Request Closure activity in the UVMClick-IACUC system. This provides the opportunity for the researcher to summarize all the activities into a final report.
Retention and Disposal of Data
In addition to informing the IACUC of the closure, the PI must store the research records for minimal required length of time in accordance with the federal regulations, UVM policy, and any additional requirements stipulated by research sponsors and/or investigators’ professional associations. Data should be retained and disposed according to the University’s Records Management and Retention Policy.
6.9 Standard Procedures and Substances
During the development of a protocol, PIs must review the Standard Library and use already created standard procedures and substances if they are available. If a Standard substance or procedure is available but does not accurately describe the intended work, a PI may make a copy of the Standard and edit as appropriate. Copies of Standards will have a Procedure Scope of “Team” and require review at the time of protocol submission.
Procedure for creating Standard substances and procedures
A procedure or substance may be added to the Standard Library after it has been reviewed and approved by the Full IACUC. IACUC staff, in consultation with the University Veterinarian and Biosafety Coordinator as needed, develop procedures and substances in UVMClick. They are first reviewed by the Policies and Procedures Subcommittee and then placed on a Full IACUC agenda for review and approval.
7.0 Who May Serve as Investigators
Eligibility requirements for conducting vertebrate animal research vary depending on the role of the researcher. Research personnel must be appropriately qualified by training and/or experience to perform their research responsibilities.
Principal Investigator
The PI is ultimately responsible for assuring compliance with applicable University IACUC policies and procedures and Animal Welfare regulations. Although the PI may delegate tasks to members of his/her research team, s/he retains the ultimate responsibility for the conduct of the study.
Because PI responsibilities involve direct interaction and supervision of the research team, the PI must be a current employee of the University who is operating within their University role to oversee the conduct of the study. PIs leaving the institution are responsible for notifying the IACUC well in advance of their departure to discuss closure or transfer to another PI.
Faculty Members
All categories of compensated faculty members may serve as PI if their College allows them to serve as PI on applications for sponsored funding administered through the University.
Non-Faculty (students/trainees)
A non-faculty researcher includes, but is not limited to, any of the following: fellow, resident, post-doctoral fellow, post-doctoral associate, post-doctoral trainee, and any student (graduate or undergraduate).
Non-faculty researchers cannot conduct animal research without having a faculty sponsor who will be the PI of record and who will be institutionally accountable for overseeing the conduct of the research activities. The faculty sponsor must be employed by the institution.
Key Personnel
Key personnel are individuals employed by the University who contribute to the scientific development or execution of a project, whether or not they receive salaries or compensation under the protocol. This includes any individual conducting experiments. Key personnel must complete required training and be listed as a member of the study team.
7.1 Principal Investigator Responsibilities
The PI’s primary responsibilities include, but is not limited to, the following;
Delegation of Responsibilities
PIs must personally perform or delegate to qualified co-investigators or research staff all of the necessary tasks to carry out their studies. Even when specific tasks are delegated, the PI remains ultimately responsible for proper conduct of the study and fulfillment of all associated obligations.
Ensure Protocol Adherence – Oversight of Research Team
The PI must provide members of the research team with sufficient oversight, training and information to facilitate appropriate procedures and protocol adherence.
Knowledge of Federal Regulations
The PI and all key personnel are expected to be knowledgeable about and comply with the requirements of each of the following:
- The Guide for the Care and Use of Laboratory Animals (PDF);
- The Animal Welfare Act;
- IACUC Policies and Procedures; and
- The terms and conditions of any research agreements (with government or private sponsors, NSF and DOD may have specific requirements).
Training of the Research Team
The PI is responsible for ensuring that the research team has appropriate training prior to and during the conduct of the study as listed in Section 7.3.
Significant Financial Interest (SFI) Disclosures
As an academic research institution, we must continually dedicate ourselves to the integrity of the research enterprise. Such conflicts are not inherently wrong, and as long as they are disclosed and appropriately managed or resolved, they do not distort and can benefit the research process. A conflict of interest may arise when a faculty or staff member has a relationship with an outside organization that puts the faculty or staff member in a position to influence the university's decisions in ways that could lead directly or indirectly to financial gain for the faculty or staff member or his or her family, or give improper advantage to others to the detriment of the University. Disclosures of SFIs are done through the UVMClick-COI software.
Provide Reports on the Progress of the Study
During the course of a research study, new information might become available. As new information becomes available, the PI is obligated to report to the IACUC.
Coverage for PI During an Absence
At the time of initial protocol submission, the PI must either designate a person to be in charge and fulfill all responsibilities for oversight of the protocol in their absence or provide a plan for coverage (one option is to cease activities during an absence – this requires IACUC notification). That person will be identified in the protocol as the designee. If a formal leave is planned, (e.g., sabbatical, medical, maternity or other official leave type) the IACUC needs to be notified, so that it may redirect protocol inquiries during that time. The responsible designee must understand the protocol and comply with the requirements as noted above.
Retention of Research Records
The regulatory mandated duration for records retention varies depending on which regulations apply to the research in question. UVM investigators need to ensure that their plan for record retention complies with the federal regulations as well as UVM Records Retention policy (PDF).
- IACUC Records means all records of communications with the IACUC and all approval documents. The RPO office retains all of these documents within the electronic system.
- Research Records refers to documentation of all observations and other data pertinent to the investigation. Responsibility to keep these records falls to the PI.
7.2 Study Team (Key Personnel) Responsibilities
This is a general guide and does not contain a comprehensive description of all the potential responsibilities of a member of the research team (key personnel).
Ensure Protocol Adherence
All key personnel must have sufficient knowledge, training, and access to the protocol to conduct appropriate procedures and ensure protocol adherence.
Knowledge of Federal Regulations
Key personnel are expected to be knowledgeable about and comply with the requirements of each of the following:
- The Guide for the Care and Use of Laboratory Animals (PDF);
- The Animal Welfare Act; and
- IACUC Policies and Procedures.
Significant Financial Interest (SFI) Disclosures
As an academic research institution, we must continually dedicate ourselves to the integrity of the research enterprise. Such conflicts are not inherently wrong, and as long as they are disclosed and appropriately managed or resolved, they do not distort and can benefit the research process. A conflict of interest may arise when a faculty or staff member has a relationship with an outside organization that puts the faculty or staff member in a position to influence the university's decisions in ways that could lead directly or indirectly to financial gain for the faculty or staff member or his or her family, or give improper advantage to others to the detriment of the University. Disclosures of SFIs are done through the UVMClick-Conflict of Interest (COI) software.
7.3 Investigator Training Requirements
General Training
A web-based training program, Collaborative Institutional Training Initiative (CITI), is used to provide general training and incorporates local content. All PIs and key personnel must take the “General Lab Animal Training – Basic Course” through CITI in addition to the species-specific course for each species that is listed on the protocol before they will be added to the roster. Training is required to be completed every three years.
General Lab Animal Course
IACUC procedures, the principles of the Three R’s, methods for minimizing animal pain and distress, facility access and logistics, use of PPE, basic animal observation and restraint, reporting animal welfare concerns, surgery/anesthesia, euthanasia, blood collection, social housing etc., are all included in the general course.
Species-Specific Course(s)
Species-specific course(s) must be taken for each species you are in contact with as described in the approved protocol. Courses available include; mouse, rat, amphibians, guinea pigs, rabbits, cattle, horses, and swine.
Documentation
Documentation of completion will appear in the UVMClick-IACUC module under that specific protocol. IACUC staff members have created a training resource and FAQ page which includes a link to the training completion dropdown to confirm that personnel have completed the relevant modules prior to addition to the protocol roster. PIs and research staff can also access this dropdown to confirm the completion of courses.
Hands-on Training
Hands-on biomethods training is offered by the OACM Veterinary Technicians and is a requirement before facility access will be granted. Training records for hands-on biomethods training is maintained by the Veterinary Technicians.
Animal Care Staff Training
Training of Animal Care staff includes review of relevant SOP's, one-on-one training by the Facility Manager, and "shadowing" of more experienced personnel. In addition, Animal Care staff all review the IACUC and the Risk Management & Safety on-line training and complete the learning-assessment tools. Other opportunities for training include staff and animal user meetings and seminars, a quarterly Animal Care newsletter, and postings to the Animal Care list-serve.
Continuing Hands-On and CITI Training
Continuing “hands-on” training for present and incoming research personnel is provided by the veterinary staff. Regularly scheduled small group sessions cover restraint, injection and sampling techniques, recognition of common health problems, aseptic technique, anesthesia and euthanasia. This training is offered to individuals on an as-needed basis following consultation with the University Veterinarian.
CITI training courses are required to be completed every three years as of November 1, 2017. Reminders letters will be sent to personnel as their training expiration date nears.
Specific Other Training
Study Team working with BioHazardous Agents
PIs that utilize hazardous agents will train those study members who will be handling the agents. Assistance may be requested from UVM’s Biosafety Officer.
Chemical Safety Training
UVM’s Environmental Health & Safety Office (EHS) offers Chemical Safety Training that covers general laboratory safety, an overview of regulations, OSHA laboratory standards and the UVM Chemical Hygiene plan. Training may also be provided by the person’s laboratory supervisor, provided that training is adequately documented. Chemical labeling and storage requirements and a copy of the UVM Laboratory Safety Audit are available on the EHS website above.
Radiation Safety Training
UVM’s Radiation Safety Office (RSO) requires that a person complete Radiation Safety Training prior to using radioisotopes or radiography in the laboratory. A certification exam is required at the completion of the course. The Radiation Safety Handbook is available online at the link above.
Animal Care Staff and Biohazards
All animal caretakers are instructed and trained by the Animal Care Facility Manager in the husbandry, sanitation procedures and precautions to be followed when working in any biohazard area. Each protocol utilizing hazardous agents requires an instruction sheet which is reviewed by the Facility Manager with the caretakers and is posted on the animal room door. The instructions on the sheet include animal and personnel safety.
Training for Students on Teaching Protocols
Animal Science majors working on protocols in which animal activities are limited to teaching are required to complete appropriate CITI trainings before they can start working with live animals. This includes the “General Lab Animal Training – Basic Course” and the species-specific course for each species they come into contact with. At the discretion of the of the course instructor, non-Animal Science major students having limited involvement with animals may take only the species-specific course. The course instructor is responsible for ensuring completion of required training for students enrolled in the class.
If the role of the student, however, involves responsibilities beyond that which is typically required of the class, such as husbandry or performing animal procedures, the student is required to take additional training as appropriate. The University Veterinarian should be contacted by the instructor for guidance.
Other Training Resources
Webinars, guest speakers and/or consultation on various relevant topics such as research models and mouse colony management are provided to the research community.
Library support for searches for alternatives to animal use and/or procedures which cause more than momentary or slight pain and distress to animals is available through the University Library Services.
Information on the following topics is also available: Levels of discomfort/distress in animal experimentation; anesthetic and analgesic drug formulary for different species; AVMA Guidelines for Euthanasia.
Self-study materials on handling and basic manipulative procedures for commonly used laboratory animals are available.
The Northern Mountain Branch of AALAS is a membership association dedicated to advancing and disseminating knowledge about the responsible care and use of laboratory animals for the benefit of human and animal health. Animal Care staff are members and attend meetings, which happen three times per year and are open to all involved in the use of animals.
7.3.1 Literature Search for Alternatives
The U.S. Department of Agriculture (USDA) and Public Health Services (PHS) mandate that every animal care and use protocol, which uses procedures that have the potential to cause more than momentary pain and/or distress to the animal (pain level D&E), document a "Search for Alternatives to Painful Procedures” for EACH potentially painful/distressful procedures proposed in a protocol.
Why do a literature search?
Beside the regulatory aspect, there are many other benefits of the alternatives search. Many researchers run literature searches when designing a study. This helps determine if the research is original or "unnecessary duplication" (which must be documented). Such searches can easily be tailored to address alternatives to potentially painful procedures. Completing and documenting this search demonstrates that the investigator will stand behind their work and that it is as humane as possible. Sometimes, the adoption of an alternative method is more economical (i.e., using an in vitro model vs. housing a colony of animals). It may provide more meaningful data by avoiding confounding factors in the experimental design such as distress in the animal model.
Ask a Librarian
PIs may ask a librarian for help conducting their searches. Library support for searches for alternatives to animal use and/or procedures which cause more than momentary or slight pain and distress to animals is available through the Dana Health Sciences Library.
- The IACUC reserves the right to require librarian assistance for protocol development at their discretion.
General Search Strategies
- Utilize different databases
- Get to know the database. Databases treat symbols, operators, and search strategies and terms differently. Before starting your search, look for tutorials, “Help” links, Frequently Asked Questions (FAQs), or advanced search options.
- Use the database thesaurus to find better terms and phrases. Some databases come with their own “thesaurus”, like PubMed’s Medical Subject Headings (MeSH; National Library of Medicine). NOTE: MeSH has to be manually updated, so the most recent terms, phrases or concepts may not be in the database yet.
- Start out searching more broadly and then slowly narrow your results by adding keywords one at a time.
- Add more and/or unique terms to your search statement
Written Narrative Guidance
- The description must include the date of the search, the database(s) searched, keywords, a summary of your findings and why you believe there are no alternatives to potentially painful procedures, and years covered.
- Summarizing your findings
- Did you determine that there are any alternatives for the procedures/species/strains that you are proposing to do/use? If a database search or other source identifies a bona fide alternative method (a potentially less painful procedure that could be used to accomplish the goals of the animal use proposal), the written narrative should scientifically justify why this alternative cannot be used. If you did not find an appropriate or plausible method, indicate that this is the only technique available for that particular purpose.
- Example
- Procedures – “Based on my years of experience in this field and periodic consultation of bibliographic sources outlined above, I believe there is no alternative to performing [insert the potentially painful/distressful procedure] in order to achieve the scientific objectives of this research. Therefore, based on the aforementioned references, this procedure is the most appropriate for conducting my research."
- This information must be updated with every revision that uses potentially painful procedures and at the de novo review.
- Regardless of the alternatives source(s) used, the written narrative should include adequate information for the IACUC to assess that a reasonable and good faith effort was made to determine the availability of potentially less painful procedures.
7.4 Occupational Health and Safety Program (OHSP)
Each institution must maintain an occupational health and safety program as an essential part of the overall animal care and use program. The OHSP must be consistent with federal, state, and local regulations and should focus on maintaining a safe and healthy workplace. UVM’s Risk Management and Safety (RMS) department oversees the University’s OHSP. Animal Care workers are contracted through Priority One Services thus falling under the Priority One OHSP, Concentra.
RMS has contracted with two medical facilities to provide occupational health monitoring for UVM employees and students. Champlain Medical, located in South Burlington, (802-448-9370) covers UVM employees and UVM Student Health Services covers students.
Risk Assessment
A specific risk assessment survey must be completed by each person listed on an active IACUC protocol. Assessments are evaluated by the appropriate entity and the risk assessment is used to determine who should receive additional follow-up and remediation (which may include pulmonary function test, use of an N95 mask, appropriate immunizations, or other precautionary measures).
For Animal Care workers, a medical evaluation is sent to Concentra for review. The Animal Care medical evaluation may include a physical exam, pulmonary function test and review of immunization status. Animal Care employees are required to have a current tetanus immunization.
Subsequent re-assessments are required triennially by submitting an updated form. If, however, employees or students have health concerns prior to any review the health care provider should be contacted.
Exceptions: Undergraduate students whose exposure to animals is limited to one hour per week per semester, research involving aquatic animals, or personnel who work only with animal products are not required to enroll in the program. Undergraduate students are made aware of the program and are provided with information about risks. Students in need of attention should contact the Student Health Center. While IACUC Board members are not required to take part in the program, they are strongly encouraged to participate if they attend semiannual inspections. Any other exceptions to required program enrollment are considered on a case-by-case basis by the UVM veterinarian.
Agent, Allergen and Other Hazard Awareness
Zoonotic agents, animal allergies and other hazards are described in the required web-based IACUC training for personnel and assessment of these risks is addressed in the risk assessment form. The triennial re-assessment form addresses changes in health status such as pregnancy or immunocompromise.
Animal Care staff members must read and sign off on a written SOP before performing any activity that may be hazardous and in addition are personally trained by the Facility Manager. Research personnel who work with infectious agents may be required to sign an informed consent document acknowledging that they are aware of the hazards and have read the relevant SOPs.
In the case of infectious agents, personnel who are immunocompromised or women who are pregnant are informed of the risk and advised to notify their supervisors.
Injury and Exposure Reporting
All research personnel or students who work with infectious agents must report any accident involving exposure to the agent to their supervisor immediately and visit the emergency room or consult with the Infectious Disease Physician on call. A plan of action is filed with the IBC and prophylactic antibiotics/vaccines may be inventoried in either the laboratory or the University of Vermont Medical Center pharmacy, depending on the risk assessment and plan for that specific agent. Serology may be done to detect changes in antibody titer and further follow-up is conducted as deemed appropriate by the Infectious Disease Physician. There are no nonhuman primate species or small ruminants currently in use as models at UVM.
Minor injuries (such as animal bites, sprains, etc.) are treated at Champlain Medical or at the University of Vermont Medical Center. University of Vermont Medical Center emergency services are readily accessible for more serious injuries should they occur.
Safety Equipment and Waste Disposal
RMS oversees laboratory safety including radioisotopes, hazardous chemical waste and use of chemical and biological agents to ensure that they are used safely and in accordance with all applicable government regulations and University policies and procedures. RMS also conducts training in the handling of hazardous chemicals, biohazardous agents and blood-borne pathogens. RMS staff members conduct regular audits of each laboratory as described in UVM's Environmental Management Plan (http://esf.uvm.edu/uvmemp/).
Animals exposed to or treated with infectious agents are maintained in rooms with double barriers in filter-topped caging or in semi-rigid isolators. When appropriate, a Class II Biosafety cabinet (BSCll) is used for husbandry and other procedures. Laboratories using infectious agents in animals must submit information about the agent and an SOP and/or BARD (Biohazardous Agent Reference Document) for working with the animals to the Animal Facility Manager. Containment practices and other information relative to the specific biological agent housed in a room then are posted on the animal room door. For chemical agents, animals' cages are marked with special cage cards and the bedding and caging are handled as appropriate to the agent. For biological agents, bedding and other animal wastes are either double-bagged in a BSCll and sealed/boxed for incineration or autoclaved prior to disposal in the regular trash. All BSCll are certified annually.
Personal Protective Equipment (PPE)
PPE appropriate to the room is indicated by signage on the door and discarded in the anteroom prior to exiting the area. PPE is provided by the animal facility for use by research personnel as well as animal care staff. This PPE may include isolation gowns and masks, gloves, shoe covers, bouffant head covers, N95 respirators, face shields and Tyvek coveralls, depending on the agent and procedures.
All Animal Care personnel are provided with work garments for daily routine wear (usually scrubs, occasionally coveralls), which are laundered by commercial arrangement (Unifirst). All Animal Care personnel are provided with rubber steel-toed boots or shoes, plastic aprons, heavy rubber gloves, ear protection, goggles, fit-tested respirators and other PPE when required. Hands are washed upon leaving an animal holding room; each room has a sink and antimicrobial soap available. When departing one building for another, caretakers are required to change to street clothes and change to clean scrubs when entering the next facility. No work-issued clothing is taken home or worn outside the animal care areas.
Animal Transportation
Animals traveling to any laboratory or procedural space outside of the animal facilities must be in filter-topped caging on a cart and covered by draping material, a box, or some other secondary container. Signage is posted in any elevators which are used for animal transport.
8.0 Funding Information
It is important for the IACUC to have funding information to allow it to conduct congruency review and to assist researchers with any specific sponsor requirements. The IACUC works with Sponsored Program personnel to ensure that an appropriate link is made between the grant application and the protocol being reviewed.
8.1 IACUC Review of Grants for Congruency
The IACUC is required to ensure that all research described in a grant application or proposal is entirely consistent with any corresponding protocol(s) reviewed and approved by the IACUC. Any discrepancies must be resolved prior to the start of the project.
Congruence will be evaluated for several important parts of protocols and proposals. The Vertebrate Animal Section and the Approach part of the Research Strategy Section are the primary areas of the grant to be compared with the protocol. Information to be reviewed include:
- General scope of work – disease area, target organs, etc.
- Species of animals
- Number of animals and sex
- Agents administered (including anesthetics, analgesics and experimental agents)
- Method to administer the agent
- Procedures to be conducted on animals
- Method of euthanasia
8.2 Just-in-Time Provision for IACUC Submissions
What is “Just-in-Time” Review
The NIH just-in-time policy allows grant applications to be submitted to NIH for peer review without prior IACUC approval. This policy has been extended by the University to all UVM grant proposals where the granting agency does not require IACUC approval at the time the proposal is submitted. Researchers should check with the Pre-Award Services Office to determine the funding agency’s IACUC approval requirements.
Process for “Just-in-Time” Review
If the sponsor accepts just-in-time IACUC review, as soon as the researcher is notified that the proposal received a favorable priority ranking from the granting agency, the protocol should be submitted to the IACUC for review. Researchers should check the IACUC submission deadlines for the next available IACUC meeting as special requests for insertion onto an agenda after the scheduled deadline may not be possible. The Committee has no means to convene IACUC outside of its normal meeting schedule. In our experience, the receipt of an award has never been affected by the date the IACUC Committee convenes to conduct the protocol review.
8.3 New Competing or Competing Renewal Grant Applications
If an investigator has received a JIT request on a new competing grant or a competing renewal, remember that it requires IACUC review and approval. The new funding source must be updated, the protocol amended as applicable, and the grant attached within the UVMClick system. The amended grant and any resulting changes to the IACUC protocol will be reviewed for approval. Sponsored Programs personnel will be unable to clear funds for release until there is an IACUC approval for the new or continuing project.
8.4 Interinstitutional Assurance Agreement
UVM researchers may arrange to carry out portions of research that involve the use of animals via an NIH-funded subcontract from an NIH-awardee (often a private company) that does not itself have an approved Animal Welfare Assurance on file with the NIH OLAW. In such situations, OLAW requires that the company apply for an "Interinstitutional Assurance," under which UVM assures that the research will be carried out under the terms of its own OLAW-approved Animal Welfare Assurance.
Interinstitutional Assurance Agreements require signatures from the private company, UVM Executive Director for Research and the IACUC Chair. If an agreement is necessary, please contact the IACUC office at iacuc@uvm.edu. The office will assist with the completion of the form and in obtaining institutional signatures.
A sample form may be downloaded at http://grants.nih.gov/grants/olaw/sampledoc/ interinstitutional_assurance.htm.
8.5 Department of Defense (DOD) supported Research Projects
All awards issued by the Department of Defense are subject to the regulatory policies and procedures of the US Army Medical Research and Material Command (USAMRMC) under Dod Instruction 3216.01 dated March 20, 2019. Researchers may not begin research activities involving laboratory animals or expend funding on such effort until applicable regulatory documents are reviewed and approved by the USAMRMC to ensure DoD regulations are met.
The USAMRMC Animal Care and Use Review Office (ACURO) is the entity within the DoD that reviews vertebrate animal research. Please contact ACURO for questions regarding their review requirements.
Scope
These requirements apply if any of the following conditions are met.
1. The research is funded by a component of the DoD (Navy, Army, Air Force, National Geospatial Intelligence Agency, National Security Agency, Defense Intelligence Agency, Defense Threat Reduction Agency, Defense Advanced Research Projects Agency, and United States Joint Forces Command);
2. The research involves cooperation, collaboration, or other type of agreement with a component of the DoD; or,
3. The research uses property, facilities, or assets of a component of the DoD.
Requirements of UVM
To be eligible to receive DoD awards UVM must be USDA registered and maintain its AAALAC International accreditation.
Requirements of the Principal Investigator
Prior to release of DoD funds to conduct research, UVM researchers must:
- Submit a new animal protocol to the UVM IACUC. DoD projects cannot be placed within a combined existing protocol. These must be separate.
- Once UVM IACUC approval is obtained, the PI will submit his/her approved protocol, UVM IACUC approval of that protocol, and a version of the animal use appendix titled “Research Involving Animals.” For guidance, visit the ACURO website at https://mrdc.amedd.army.mil/index.cfm/collaborate/research_protections/acuro
- PIs may be requested for additional information for example statements that animals are legally obtained from suppliers licensed by the USDA or capture and use permits.
- Once ACURO has approved the protocol, the researcher needs to upload the ACURO approval document as a comment in the UVM IACUC Click approved protocol record.
Allow two months for Government regulatory review and approval processes for animal studies.
For additional information, send questions to ACURO at ACURO@amedd.army.mil.
Release of Funds
To allow Sponsored Projects Administration to release research funds, the PI must document that he/she has IACUC approval from both IACUCs. To accomplish this, the PI must upload the DoD IRB approval document to the UVM IACUC Click approved protocol record as a comment.
Ongoing DoD Oversight
Any substantive modifications to an approved protocol must be reviewed by the UVM as well as the DoD IACUC prior to the change in activities. A list of substantive changes can be found under news and announcements dated July 1, 2020 “ACURO Protocol Change Guidance”.
9.0 Monitoring
In addition to the semi-annual inspections outlined in Section 15.0, UVM has a post-approval monitoring program and receives external visits to assess compliance.
Post-Approval Monitoring (PAM)
The Monitoring and Compliance Specialist selects protocols (1-3) per month to review. Using the below Risk Assessment for PAM Visits chart, at least one “high” level protocol will be chosen per month. Protocols are also selected for cause or as a result of a request by veterinary staff or lab personnel. The protocols are reviewed to ensure research is being conducted in accordance with what is written and approved.
PAM is an opportunity for investigators to request any help or information they may need regarding IACUC Office processes. The resultant report is maintained by the IACUC Office and provided to the IACUC at a Full convened meeting. Follow-up visits may be conducted to document that any findings highlighted during a visit have been rectified.
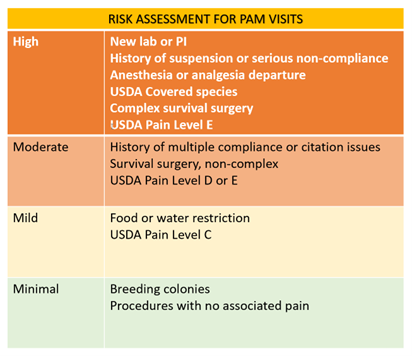
USDA Inspections
Annually, an Animal Welfare Officer from the USDA will inspect the animal facilities and may inspect individual labs. The inspector will meet with the University Veterinarian or a designee who will escort the Animal Welfare Officer through the facilities. The USDA inspector will also review IACUC protocol records.
AAALAC International Site Visits
AAALAC International is a private, nonprofit organization that promotes the humane treatment of animals in science through voluntary accreditation and assessment programs. At the request of UVM, AAALAC International visits UVM every three years to assess our program.
10.0 Semi-Annual Inspections and Program Review
Revised 11/27/23
Semi-Annual Inspections
The IACUC inspects all the institution’s animal housing facilities at least once every six months using the “Guide”, the “Ag Guide” and the Animal Welfare Act and Regulations (AWAR) for guidance. All animal housing facilities are inspected, including: satellite facilities (containment areas outside of the central animal facilities where non-USDA-covered animals are housed for more than 24 hours and cared for by researchers), areas in which surgical or behavioral manipulations are performed, animal study areas (locations where USDA-covered animals are held for more than 12 hours), and standard holding facilities. As of 2023, facility inspections are conducted on a staggered schedule semiannually. Each area will be inspected no more than 6 months between visits, including a 30 calendar day flexibility per NOT-OD-21-164 that avoids forward drift of inspections year to year.
The PHS Policy Footnote 8 and 9 C.F.R. § 2.31(c) (3) of the AWA/R allow IACUCs to determine the best means of evaluating the institution’s facilities. For areas housing non-Animal Welfare Act (AWA)-regulated species, the IACUC may use as few as one qualified individual or ad hoc consultant, who need not be an IACUC member or institutional employee, to conduct the facility inspections. Qualified individuals should have training and a working knowledge of the PHS Policy, Guide, and the AWARs to appropriately evaluate the facilities and identify deficiencies and animal welfare issues.
For areas housing AWA-regulated species, the IACUC may use subcommittees composed of at least two committee members and may also invite ad hoc consultants to assist in conducting the inspections. IACUC members involved in these inspections are not required to inspect together and may each inspect different parts of the facility.
Inspection teams are formed based upon the IACUC members’ requests and schedule availability. Each member of the IACUC is expected to participate in at least one of the two semiannual inspections each year. All IACUC members are allowed to participate in any portion of any inspection. A researcher or facility manager should not be the sole IACUC inspector for their own lab or facility. Standard operating procedures are made available to the inspection team and the team is briefed about the findings of the previous inspection and the steps taken to address any problems or concerns, if applicable.
Draft reports of the inspections are prepared according to PHS Policy criteria and are submitted to the full committee for review and discussion. The IACUC identifies departures from the PHS Policy and the Guide by referring to those documents as well as the AWA regulations and the Guide for the Care and Use of Agricultural Animal in Research and Teaching. Approved departures are included in the semi-annual report. Deficiencies noted during the semi-annual inspection are categorized as minor or significant and corrective action plans are included in the semi-annual report to the Institutional Official (IO). The IACUC staff, in cooperation with the University Veterinarian, monitors progress on the corrective actions to ensure completion according to the timeline included in the plan. Any minority views are documented and included in the report.
Program Review
The IACUC conducts a program review at least once every six months to evaluate the institution's program for the humane care and use of animals covered by this assurance, using the “Guide” the “Ag Guide” and the AWA/R as a basis for evaluation. The IACUC conducts its semi-annual program evaluation by:
- Delegating to the Policy and Procedure Subcommittee the initial review of institutional policies for completeness and consistency with all applicable guidelines, utilizing the OLAW Semiannual Program and Facility Review Checklist.
- Reviewing reports of protocol monitoring activity to determine if any issues need to be incorporated into the program review.
- Placing special emphasis on animal health and well-being during these inspections.
- Integrating issues raised in the IACUC meetings, the IACUC Policy and Procedure Sub-Committee meetings, as well as regular meetings between the IACUC staff, the IACUC Chair and the Office of Animal Care Management into the program evaluation process.
- After review and inspection, a written report (including minority views) will be reviewed and signed either in person or electronically by the majority of IACUC members. The report will be provided to the IO.
11.0 Reporting Requirements
Revised: 8/28/23
Each set of regulations has specific reporting requirements as outlined below. The Research Protections Office (RPO) is responsible for submitting these reports.
Office of Animal Welfare (OLAW)/NIH
Annual Reporting
This Institution's OLAW reporting period is October 1 - September 30. The RPO will submit an annual report to OLAW on or before December 1st (but after September 30) of each year. The report will include as required:
- Any program changes since the last report
- Dates of semiannual evaluations for both the program and the facilities
- Any minority views filed by members of the IACUC during the reporting cycle
- Change in IO
- Change in the IACUC membership
- Any change in the accreditation status of the Institution (e.g., if the Institution obtains accreditation by AAALAC or AAALAC accreditation is revoked)
Off-Cycle Reporting
UVM as a Public Health Service awardee institution is required to promptly provide OLAW, through the IACUC and IO, a full explanation of the circumstances and actions taken with respect to:
1. Any serious or continuing noncompliance with the PHS Policy;
2. Any serious deviations from the provisions of the Guide; or
3. Any suspension of an activity by the IACUC
Prompt reports are required for incidents that have a negative impact on animal health and well-being, while other incidents may be submitted in the annual report.
UVM is required to report for projects federally funded as described in our Animal Welfare Assurance. We are not obligated to report when there is no federal funding. The process for investigating and managing serious or continuing noncompliance or serious deviations is the same regardless of funding source. The RPO is responsible for submitting these reports. All personal and sensitive information will be redacted from reports prior to submission.
Renewal
The Institution’s Assurance with OLAW is required to be renewed every four years. Prior to the expiration date, RPO will submit the Domestic Animal Welfare Assurance (Domestic Assurance) using the sample domestic assurance template and email it to olawdoa@mail.nih.gov
United States Department of Agriculture (USDA)/Animal and Plant Health Inspection Service (APHIS)
Annual Research Facility Report
Animal Welfare Act (AWA) regulations require all research registrants to file an Annual Report of Research Facility (APHIS Form 7023) with the USDA’s Animal Care program. This annual report documents activities and animal usage for the reporting period of October 1 through September 30. All annual reports are required to be submitted to USDA Animal Care by December 1st of each year through their online reporting system. Even if no animals were held or used during the reporting period, an annual report must be completed and submitted.
Off-Cycle Reporting
It is not a requirement to report serious adverse events and all incidents of protocol noncompliance to USDA. In addition to the above standard reports, the only other additional required reports are:
- Change in operations (9 CFR 2.30 (c)(1))
- Protocol suspension (9 CFR 2.31 (d)(7))
- Uncorrected significant deficiencies from a semi-annual inspection 9 CFR 2.31 (c)(3))
All personal and sensitive information will be redacted from reports prior to submission.
Triennial Registration Renewal
As of December 27, 2021, the USDA removed the administrative requirement to update the research facility registration every 3 years, as it is a duplicative requirement. Facilities are already required to notify APHIS of any change in the name, address, or ownership, or other change in operations affecting its status as a research facility, within 10 days after making such change.
AAALAC Reporting
Annual Reporting
Each year in mid-December, the AAALAC International office makes available the online Annual Report form. AAALAC International’s Rules of Accreditation require that the Institution submit an Annual Report to maintain accreditation. The annual reports are submitted by the RPO in January.
Annual Reports provide notification of any:
- Key personnel contact changes
- Changes in physical areas of supporting animal care and use
- Actions taken in response to Suggestions for Improvement (SFIs)
- Organizational structure changes
- Animal usage
- Protocol violations which had the potential to compromise animal welfare
- Animal use not approved by the IACUC or comparable oversight body
- Significant adverse events not previously reported as required by the Rules of Accreditation
Off-Cycle Reporting
The following are additional required reports.
- Adverse events to be reported promptly:
- Unexpected animal deaths
- Natural disasters
- Significant animal rights activities
- Inappropriate euthanasia techniques and/or failure to confirm euthanasia
- Allegations/complaints/reports regarding animal welfare concerns
- Lack of veterinary care
- OLAW/USDA investigations
- Other information to be reported promptly:
- Changes in unit contact (please include degree, title, address, phone and fax numbers, and email)
- Changes in facility size, location, name if site visit pending before Annual Report is to be submitted
- Adverse Event Reports following the IACUC’s Adverse Event Assessment and Reporting Plan. All personal and sensitive information will be redacted from reports prior to submission.
12.0 Noncompliance Policy
Revised 03/10/23
It is the responsibility of the Institutional Animal Care and Use Committee (also referred to as IACUC) in accordance with PHS Policy.IV.B.1-8 and 9 CFR.2.31 (c) (1)-(8) and 2.31 (5) (6) & (7) to review reports of noncompliance with animal care and use within the institution. To exercise this authority the IACUC is empowered to inspect laboratories, procedure areas, animal housing areas, and to sequester research or training records. The IACUC may receive reports in several different ways including external complaints, internal complaints, Concerns through UVMClick, random and directed site visits, and investigator self-reporting. The IACUC encourages faculty, staff, and/or students to report instances of noncompliance, especially when animal or human health and welfare is in question.
This document describes the procedures for handling these matters. This policy is not all encompassing, and the IACUC reserves the right to use its discretion in individual cases.
Definitions
Noncompliance is defined as the conduct of research in a manner that deviates from the approved protocol or disregards or violates federal regulations or institutional policies. Noncompliance may result from actions or omissions by study personnel, and can range from relatively minor or technical deviations to serious deviations that threaten animal welfare.
Serious Noncompliance is defined as noncompliance that, in the judgment of the IACUC, potentially increases the risk of harm to animals.
Continuing Noncompliance is defined as a pattern of noncompliance (recurring or ongoing) that, in the judgment of the IACUC, may indicate an underlying deficiency in knowledge of the regulations or IACUC requirements or an unwillingness or inability to comply with these regulations/requirements.
General Review Procedures
The investigation of potential noncompliance begins when the IACUC becomes aware of potential noncompliance. This may include an allegation (unproved assertion) of noncompliance, a self-disclosure of noncompliance, or any other indication that noncompliance may have occurred. The process for the review of potential noncompliance involves initial administrative review, followed by an inquiry/fact finding process if indicated. Once complete, the IACUC makes a determination as to whether the noncompliance is serious, continuing, or neither. The IACUC determination will be documented in a summary report that contains a corrective action plan in cases of serious or continuing noncompliance. This process is detailed below, however at any point in the review process, the IACUC designee may at their discretion:
- Recommend intervention for the safety of animals
- Recommend the suspension of research activities
- Inform, involve, and/or provide salient documents to the PI, members of the research team, the Department Chair, Dean, legal counsel, or Institutional Officials, as appropriate
- Initiate reporting per federal regulations
- Initiate a monitoring visit
- Recommend immediate corrective actions
Process of Noncompliance Review and Determination
Initial Review of Allegation or Indication of Noncompliance: When there is an allegation or indication of noncompliance, the first step is an administrative review to determine if, in the judgement of the person(s) conducting the review, there is the potential for serious or continuing noncompliance. The initial review may be conducted by the RPO Director, RPO Assistant Director(s), an IACUC Chair (Associate Chair or Chair), University Veterinarian or another Institutional Representative. Allegations/indications which are determined to have no potential to be serious and/or continuing noncompliance are resolved with either no follow-up (i.e. when an allegation or indication has no merit) or directly with the PI.
Inquiry/Fact Finding Process: If it is determined that the noncompliance has the potential to be serious or continuing or if questions remain following the initial review, then an inquiry (fact finding) process will begin. The particular circumstances of the noncompliance will determine when the fact finding begins and when the committee is briefed. The fact finding may be conducted by any IACUC designee including a sub-committee or subcommittee member, the RPO Director, Assistant Director(s), an IACUC Chair (Associate Chair or Chair) or other Institutional Representatives. The IACUC may be briefed at any point throughout the fact finding process, as deemed appropriate by the designee. The fact finding process continues until the designee has arrived at a recommendation of determination (i.e. serious noncompliance and/or continuing noncompliance, or neither). A fact finding report is then prepared and includes the recommendation of determination and draft corrective actions. This fact finding report will be shared with the PI, and if applicable, other person(s) involved. All parties will be provided an opportunity to respond to any factual inaccuracies within the report before the committee deliberates.
Deliberation by the IACUC: At a convened meeting, the IACUC will consider all available information and make a determination as to whether the fact finding revealed serious noncompliance and/or continuing noncompliance, or neither. The following factors will be taken into consideration by the IACUC or designee in making their initial determination as to whether the noncompliance is serious and/or continuing noncompliance. As each situation is unique, the indicators of noncompliance that are important in one case may not be relevant in other cases.
Factors in the Determination of Serious Noncompliance:
- Level of risk or potential risk to animals
- Severity of violation of the research process
- Frequency or number of minor deviations or errors
- Intent
- Threat to integrity of the IACUC review processes and requirements for the protection of animals (i.e. falsification of IACUC documents)
- Other factors that, in the judgement of the IACUC or designee, are relevant to the situation being reviewed.
Factors in the Determination of Continuing Noncompliance:
- Similarity of noncompliance to previous deviations and/or noncompliance within the same protocol.
- Similarity of noncompliance to previous deviations and/or noncompliance in other protocols conducted by the investigator.
- Likelihood that instances of noncompliance will continue without intervention
Final Determination of the IACUC: If, in the judgment of the committee, the noncompliance is neither serious nor continuing, this determination will be shared with the PI. If, in the judgement of the committee, the noncompliance is serious and/or continuing, the designee will prepare a summary report including the IACUC’s determination and an approved corrective action plan. This report will be shared with the PI, who will be given 14 days to review it before it becomes final.
Development of Corrective Action Plans:
The IACUC/designee will develop a proposed plan for corrective actions based on the information gathered during fact-finding and input from the PI and/or other affected individuals. The proposed plan may:
- Require no further action
- Require minor corrective actions to achieve compliance
- Require additional education
- Require the investigator and/or other affected individuals to develop and implement procedures to prevent recurrence
- Review internal departmental or institutional mechanisms and systems for opportunities to prevent recurrence or similar occurrences by others
- Require additional oversight (e.g., by other faculty member or department process)
- Require more frequent IACUC reviews
- Require internal monitoring visits or monitoring plans
- Suspend or terminate individual protocols
- Restrict researcher’s research activities
Requests for Reconsideration
A PI may request a reconsideration of the IACUC’s determination. Requests must be limited to claims that either (1) the process was faulty, resulting in considerable risk that the outcome was incorrect; or (2) that the findings and/or corrective actions imposed by the IACUC were excessive or unjustified. The written request must be submitted within 5 days of receipt of the summary report and must specify the nature of any claimed procedural error or the perceived unfairness of actions taken. Reconsiderations will be conducted by an IACUC Chair (Chair or Associate Chair), or Designee. The reconsideration process will result in one of three outcomes: the summary report will stand and it will become final, the summary report will be modified and it will become final, or further investigation is necessary and will be initiated.
Required Reporting
When noncompliance is determined to be serious and/or continuing, the final report will be forwarded to federal regulators if required, AAALAC, the institution’s accrediting body, and to applicable Institutional Officials, the Departmental Chair, the Dean, and sponsors, if applicable. The RPO is responsible for submitting these reports and will safeguard investigator's identities.
Guiding Principles for Noncompliance Review
Protection of Animals: The University of Vermont is committed to the humane care and use of animals in activities related to research, testing and teaching.
Fairness: The IACUC strives to maintain a review that is impartial and honest, free from self-interest, prejudice, or favoritism.
Communication: The committee will communicate with the PI during the review process at points determined to be appropriate by the IACUC designee.
Confidentiality: All IACUC discussions and documents regarding a situation of noncompliance are considered sensitive and will be handled in a confidential manner and in accordance with state and federal regulations. The IACUC cannot, however, guarantee complete anonymity to informants or witnesses. Confidentiality will be maintained to the extent possible to protect privacy and prevent retaliation, while still allowing for a full and fair review. Information may be shared, as described above under Required Reporting.
Conflict of Interest: Any IACUC member who feels that they have a conflicting interest must recuse themselves from reviewing the issue of noncompliance. IACUC members who are also listed as key personnel on the protocol(s) will not participate in the review but may be asked for information.
Procedures: In addition to what has been stated within this policy, the Committee will follow all applicable procedures that are outlined within the Committee Operating Procedures document.
13.0 Projects in which Animal Use is Limited to Teaching Activities
The UVM IACUC as partnered with Animal Science department leaders to create a master protocol that includes all Animal Science projects that are solely used for teaching purposes that do not include procedures that cause more than momentary pain or distress.
Faculty developing a protocol that meet those criteria must be in contact with the IACUC to add your new class to the master protocol.
14.0 Use of animal tissues
UVM is committed to reducing the number of animals used in research. Tissue sharing is strongly encouraged as this allows investigators to collect fresh tissue without euthanizing additional animals.
For Investigators Donating Tissue
UVM investigators who must euthanize their animals under an approved protocol for experimental requirements are encouraged to donate post-mortem tissue to other UVM investigators and can do so without prior IACUC review and approval. It is the donating investigator’s responsibility to communicate any biosafety issues associated with the tissue.
If UVM investigators wish to share tissue outside of UVM they should contact UVM’s Office of Technology Transfer to determine if a Material Transfer Agreement is recommended prior to releasing the tissues. Go to this link for more information https://www.uvm.edu/spa/material-transfer-agreements-mta
For Investigators Receiving Tissue
UVM investigators who wish to use leftover tissue from other UVM IACUC protocols can do so without submitting a separate tissue protocol. The IACUC does not need to review this activity as all live animal use protocols at UVM have been reviewed and approved previously. The use of the tissue activity no longer requires review.
UVM investigators wishing to use tissue from institutions outside of UVM should obtain their tissues only from acceptable sources. Acceptable sources would be other institutions that have obtained IACUC approval for an animal use protocol, preserved specimens from corporate labs, or a USDA inspected slaughterhouse. If the materials come in with a Material Transfer Agreement, UVM researchers need to work with the Office of Technology Transfer. Go to this link for more information https://www.uvm.edu/spa/material-transfer-agreements-mta.
15.0 IACUC Approved Policies
Individual policies that have been approved by the IACUC
15.1 Agricultural Animals in Research and Teaching
Policy and Procedures for Use of Agricultural (Farm) Animals In Research and Teaching
Scope
In accordance with the Animal Welfare Act, the IACUC has the authority to regulate farm animals when used for biomedical research and teaching purposes. UVM extends that commitment to farm animals when used or intended for use as food or fiber, or when used or intended for use in agricultural research, such as, improving animal nutrition, breeding, management, or production efficiency, or for improving the quality of food or fiber. The IACUC uses the “Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 4th Edition,” for establishing appropriate standards for use of agricultural animals.
The majority of farm animal research at UVM is food and fiber and takes place at the Miller Dairy Center Complex, The Horticulture Research Center or the Morgan Horse Barn in Weybridge. Biomedical research in farm animals is restricted to the Ungulate Facility. Other facilities may be used only if such facilities meet required standards of the applicable Guides or regulations.
Policy, Procedures and Guidelines
When a UVM faculty member contracts or arranges to utilize animals on a commercial farm for purposes of research or teaching, the IACUC must determine that the specific farm is committed to providing a high standard of animal welfare. Prior to IACUC submission, the UVM PI must work with the farm owner or manager to obtain documentation of the farm’s commitment to responsible animal care. The National Dairy Farm Standards is one example of documentation.
The Vice President for Research has delegated the following roles and responsibilities for oversight of the use of agricultural animals:
| ENTITY | ROLES/RESPONSIBILITIES |
|---|---|
| IACUC | The IACUC is responsible for overseeing the program for the care and use of agricultural animals in research and teaching. Specifically, the IACUC is responsible for 1) the review, approval and oversight of protocols involving animals in agricultural research and teaching, (2) ensuring that standard operating procedures are present for routine care and husbandry, and (3) inspecting and approving the facilities where animals are housed and research is taking place. |
| University Veterinarian | The University Veterinarian is responsible for the oversight of health care and routine care and husbandry for agricultural animals. When the University engages third parties to provide such care, these arrangements should be specified in the IACUC protocol and subject to the University Veterinarian’s or their designee’s oversight. At the discretion of the University Veterinarian and the IACUC, this oversight may include announced or unannounced visits to the cooperating farm. |
| College of Agriculture and Life Sciences | Agricultural animals that are held on UVM facilities are principally a College resource. The College is responsible for animal ordering and inventory of animals for research and teaching activities when the animals are housed in University facilities (e.g. the Miller Dairy Research Center, Horticulture Research Center, and the Morgan Horse Farm). The College is responsible for providing the resources for maintaining the animals in accordance with established standards for health and welfare. Training of research personnel, as well as ensuring they have access to the UVM Occupational Health Program, is the responsibility of the PI. |
| OACM | The Office of Animal Care Management is responsible for ensuring that animals used for biomedical research purposes are ordered from approved vendors with clean health reports and are cared for in OACM-managed facilities according to OACM standard operating procedures. |
| Large Animal Veterinarian | Large animal veterinarians, either employed by the University or a third party, are responsible for general health care and monitoring of animals used in research and teaching protocols. Collaborator farms must demonstrate a valid veterinarian-client-patient relationship with a licensed veterinarian. |
| Commercial farms collaborating with UVM researchers | Prior to approval of the research, commercial farms must provide documentation of responsible animal care to the Principal Investigator and execute a Memorandum of Understanding, which is approved by the IACUC. |
| Teaching and /or Research Faculty: | It is the PI’s responsibility to ensure appropriate monitoring of animals specific to their protocol. Farm workers associated with the research animals need to be added to specific protocols, and trained on the principles of agricultural research animal care and use. |
https://nationaldairyfarm.com/farm-animal-care-version-4-0/
15.2 Anesthesia Machine Certification Policy
Adapted from OACM’s Maintenance of Anesthesia Machine with F/Air Canister Filter SOP
Policy
Each piece of equipment involved in the delivery of inhalant anesthetics and removal of waste gasses must be evaluated regularly to ensure its proper function and integrity. Tubing, hoses and rubber items are common areas of concern and should be periodically checked and replaced as needed.
Vaporizers:
- Anesthesia machines must be maintained in good working condition to assure optimal agent delivery in a safe manner.
- Vaporizers will be certified at least once a year by TSP (Technical Service Program) or as needed.
- Vaporizers must have the certification date affixed to the machine after each service.
Waste Gas Scavenging Systems:
Waste gas scavenging systems, both active and passive, must be maintained according to manufacturer’s instructions to ensure that they are working properly. Visit https://www.uvm.edu/riskmanagement/anesthetic-gas-use for more information.
Low profile nosecones are recommended for use with rodents as they are designed for a tighter fit when used with mice and rats. This decreases the likelihood of anesthesia fumes escaping.
Note: Low flow anesthesia machines are subject to different certification standards. Default to the manufacturer’s recommendations.
15.3 Animal research involving hazardous material
All hazardous materials including chemical, biological, or radioactive agents to be administered to animals must be defined in the animal use protocol. The Principal Investigator (PI) of the research project is responsible for evaluating and indicating the potential human and animal health hazards of the agents being used in a research project and to obtain prospective approval from the appropriate review Committees prior to initiation.
Some hazardous materials are strictly controlled by federal, state and local regulations. Therefore, the University of Vermont has established specific committees and offices composed of professional staff and faculty with expertise in handling these hazardous materials. These committees/offices include:
1) Environmental Health and Safety Office, (6-5400)
2) Institutional Biosafety Committee, (6-5040)
3) Radiation Safety Office, (6-2570).
It is the IACUC’s expectation that the appropriate biosafety committee or office will review the intended hazardous material use and provide approval after all safety issues have been addressed by the PI. Personnel identified as working with hazardous agents must complete appropriate training as prescribed by the relevant Committee prior to working with any hazardous agent. The IACUC will not release animal use protocol approval until all applicable approvals are in place.
Go to the IBC Researcher Manual for definitions of some of the hazardous materials listed below.
Radioactive Materials or Radiation
Any animal research protocol involving the use of specified radioactive materials and X-ray procedures must be reviewed by the Radiation Safety Office in addition to the IACUC. It is the Principal Investigator’s responsibility to submit the appropriate forms for review to the Radiation Safety Office as well as to complete the appropriate sections in the animal use protocol form.
Infectious Agents (pathogenic to human or animal)
Review of any animal research protocol involving the use of Infectious Agents must be reviewed by the Institutional Biosafety Committee in addition to the IACUC. It is the Principal Investigator’s responsibility to submit the appropriate forms for review to the Institutional Biosafety Committee as well as to complete the appropriate sections in the animal use protocol form.
Biotoxins
Review of any animal research protocol involving the use of Biotoxins must be reviewed by the Institutional Biosafety Committee in addition to the IACUC. It is the Principal Investigator’s responsibility to submit the appropriate forms for review to the Institutional Biosafety Committee as well as to complete the appropriate sections in the animal use protocol form.
Known or suspect chemical carcinogens or mutagens
Review of any animal research protocol involving the use of chemical carcinogens or mutagens must be reviewed by the Environmental Health and Safety Office in addition to the IACUC. It is the Principal Investigator’s responsibility to submit the appropriate forms for review to the Environmental Health and Safety Office as well as to complete the appropriate sections in the animal use protocol form.
Recombinant DNA
Review of any animal research protocol involving the use of Recombinant DNA must be reviewed by the Institutional Biosafety Committee in addition to the IACUC. It is the Principal Investigator’s responsibility to submit the appropriate forms for review to the Institutional Biosafety Committee as well as to complete the appropriate sections in the animal use protocol form.
Tumors, Cells, Sera or Other Body Fluids
Tumors, cells, sera or other body fluids derived from humans and used in live animals may potentially carry disease that could infect research and animal care personnel. If any of these materials are to be used, they need to be described as a biohazard in the Animal Care and Use Protocol and may require review by the Institutional Biosafety Committee.
Tumors, cells, sera or other body fluids derived from animals are potential sources of animal
and/or human disease. There is a special concern with rodent viruses that could spread throughout the animal facilities (and have done so at other institutions). If you are using any of these agents, describe in the Animal Care and Use Protocol and discuss with the University Veterinarian.
Hazardous Waste
Animal wastes and carcasses that may be contaminated with hazardous materials must be carefully managed to avoid human exposure or damage to the environment. Hazardous waste material must be disposed of in accordance with the guidelines established by the Committees listed above.
Principal Investigator’s Responsibilities
The Principal Investigator will provide those personnel under his/her supervision with knowledge of biohazards to which they may be exposed and safety procedures to be followed. The PI must:
- Be knowledgeable of good laboratory safety practice and adopt and model a positive safety attitude.
- Make available to the laboratory staff copies of protocols that describe potential biohazards and the precautions to be taken. These protocols as well as biosafety concerns should be produced in the form of a standard operating procedure (SOP) for the work which is reviewed and approved by the appropriate Committee or safety personnel.
- Provide laboratory staff with both formal and informal instruction and training in the practices and techniques required to ensure safety and document that training appropriately.
- Inform the laboratory staff of the reasons and provisions for any precautionary medical practices (e.g., with health history evaluation, risk assessment and when indicated medical examinations and vaccinations, etc.) and document informed consent by laboratory personnel.
- Oversee the performance of staff to ensure that required safety practices and techniques are employed.
- Make available to the laboratory staff, copies of the emergency plans covering accidental spills and personnel contamination resulting from biohazardous research.
Office of Animal Care Management Responsibilities
The Office of Animal Care Management has the same responsibilities as above for the Animal Care Management staff.
15.4 BLOOD COLLECTION IN RESEARCH ANIMALS
The volume and frequency of blood collection in research animals can affect animal welfare and produce physiological variables. This is particularly true in small animals, such as rodents, birds, and small fish.
Blood volume is rapidly restored after blood collection, but a period of time is needed for blood constituents (red blood cells, platelets, clotting factors, etc.) to be regenerated by the body. One standard for acceptable blood sampling volume (in mL) is 1% of an animal’s body weight (in grams) every 14 days. Using this standard, 0.3 mL may be collected every two weeks from a 30-gram mouse. Another standard for acceptable blood sampling volume (in mL) is 10% of total blood volume, which is approximately 7% of body weight (in grams). Using this standard, 0.21 mL may be collected every two weeks from a 30-gram mouse.
Recent literature (Raabe et al, 2011) indicates that regeneration time in mice may be faster than 14 days, and that removal of a higher percentage of total blood volume is well tolerated, suggesting that 15% of total blood volume may be safely removed every 7 days. Using this guidance, 0.315 mL may be collected every week from a 30-gram mouse.
Regardless of the frequency and volume of blood removal, the overall health status of the animal should be monitored after blood collection to avoid anemia and other adverse physiological effects.
Common blood collection methods in unanesthetized rodents:
Submandibular vein (Mouse): The mouse is restrained by gripping the skin over the back of the neck to pull the facial skin taught over the mandible. The submandibular vein is punctured at the posterior border of the mandible using a bleeding lancet or tip of a 21-25G sterile hypodermic needle held perpendicular to the face. Blood is collected directly into a tube. Once sufficient volume has been collected, release of manual restraint usually stops the bleeding. If bleeding continues, apply pressure to the vein for 20 seconds.
A full description of this technique, with photos, is available at http://www.labanimal.com/laban/journal/v34/n9/abs/laban1005-39.html
Lateral saphenous vein (Mouse, Rat): The animal is restrained by placing it head-first into a conical tube, decapicone, or acrylic restraint device. The hind limb is extended over the top edge of the tube, applying pressure above the knee and making the lateral saphenous vein visible just below the knee. The sampling site may be shaved, and the vein is punctured with a lancet or 25G sterile hypodermic needle. Once sufficient volume has been collected, release of pressure against restraint device usually stops the bleeding. If bleeding continues, apply pressure to the vein for 20 seconds.
Tail tipping (Mouse, Rat): The animal is restrained manually or by placing it head-first into a conical tube, decapicone, or acrylic restraint device. The tip of the tail (<2mm) is cut with a sterile blade or sharp scissors to produce a small volume of blood. Bleeding is stopped by applying gentle pressure to the tip of the tail for 10 seconds.
Lateral tail vein (Mouse, Rat): The animal is restrained by placing it head-first into an acrylic restraint device and withdrawing the tail from an opening. Warming the tail with a heat lamp or warm water bath enlarges the vein and facilitates sampling. The lateral tail vein is more easily visualized in a light-colored rodent. The tail is stabilized and a 25G needle is inserted into the vein with bevel facing upward. Bleeding is stopped by applying gentle pressure to the tail for 10 seconds.
Common blood collection methods in anesthetized rodents:
Retro-orbital sinus (Mouse): The mouse is anesthetized, placed in lateral recumbency, and the head secured and eyelids held open with the non-dominant hand. A capillary tube is inserted at a 30-degree angle from either the medial or lateral canthus toward the sinus behind the eyeball. Once sufficient volume has been collected, the capillary tube is removed and the eyelids held closed for a few seconds. Ophthalmic ointment may be applied to the eye prior to recovery from anesthesia.
Training and observation is required prior to performing this method on experimental animals. Due to the risk of ocular damage, repeated bleedings should use alternate eyes and be limited to two collections per mouse per week and six collections per mouse total. The animal should be euthanized immediately if the eye is injured as a result of retro-orbital bleeding.
Cardiac puncture (Mouse, Rat): This method should be used only as a terminal procedure for blood collection under deep general anesthesia. The anesthetized animal is placed in dorsal recumbency and the position of left ventricle identified by maximal heartbeat. A needle is inserted between the ribs and blood withdrawn directly from the heart. The animal must be verified dead after exsanguination.
Reference: Raabe, BM, Artwohl, JE, Purcell, JE, Lovaglio, J, Fortman, JD. (2011) Effects of weekly blood collection in C57BL/6 mice. JAALAS 50(5): 680-685.
15.5 DEATH AS AN ENDPOINT - DEFINITIONS, POLICIES AND GUIDELINES
DEFINITIONS: Death as an endpoint refers to projects in which the animals’ induced death is required as a measured data point. It does not refer to projects in which the animals will be euthanized for tissue collection or project termination.
Moribund is defined as the clinically irreversible condition leading inevitably to death. Animals are moribund if they are unconscious or show no response to external stimuli such as a human hand introduced to the cage.
POLICIES AND GUIDELINES
The routine use of death as an endpoint is discouraged. Researchers should always consider alternative endpoints and use those alternatives when the research objectives allow. According to the Guide, "the use of humane endpoints contributes to refinement by providing an alternative to experimental endpoints that result in unrelieved or severe animal pain and distress, including death”. The humane endpoint is defined as “the endpoint at which pain or distress in an experimental animal is prevented, terminated or relieved.” Preemptive euthanasia can help prevent unnecessary pain and distress. Examples of humane endpoints are listed in Table 1 below. Investigators must perform euthanasia on all moribund experimental animals unless there is scientific justification that euthanasia would invalidate experimental data collection. All "death as an endpoint" protocols without relief of pain or distress are identified as USDA Pain Level E.
If euthanizing a moribund animal would invalidate the study, the scientific justification for using death as an endpoint must be provided in writing as part of the animal care protocol and must be approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC). Investigators who receive approval from the IACUC to use death as an experimental endpoint must also agree to the following:
1. Written records of all monitoring sessions, indicating the time of the observations, the person observing the animals, and any observations such as the number of animals evidencing clinically abnormal behavior and the number of animals found dead, must be maintained and made available to the Office of Animal Care Management and the IACUC.
2. Animals must be monitored at a minimum of twice daily and any animals evidencing clinically abnormal behavior must be removed from group housing situations and housed individually with easy access to food and water.
3. Use the minimum number of animals necessary to achieve statistical significance as estimated by a sample size calculation and to use alternative endpoints other than death whenever possible.
TABLE 1. Montgomery’s endpoint criteria for the euthanasia of moribund animals (Taken from: Montgomery, C.A., Jr. Oncological and toxicological research: Alleviation and control of pain and distress in laboratory animals. Cancer Bulletin; 42(4):230-237, 1990).
- Rapid weight loss (15-20% in less than a week).
- Extended period of weight loss (progressing to emaciated state).
- Spreading area of alopecia caused by disease.
- Rough hair coat, hunched posture, distended abdomen, or lethargy, especially if debilitating or prolonged (3 days).
- Diarrhea, especially if debilitating or prolonged (3 days).
- Coughing, rales, wheezing, and nasal discharge.
- Distinct icterus and/or anemia.
- Rapid growth of mass or masses or clinical signs of neoplasia.
- Central nervous system signs such as head tilt, tremors, spasticity, seizures, circling, or paralysis or paresis, especially if associated with anorexia.
- Frank bleeding from any orifice.
- Markedly discolored urine, polyuria, or anuria.
- Persistent self-induced trauma.
- Lesions interfering with eating or drinking.
- Clinical signs of suspected infectious disease requiring necropsy for diagnosis.
- Other clinical signs judged by experienced technical staff to be indicative of moribund condition.
15.6 Euthanasia methods definitions, policies and guidelines
DEFINITIONS:
- Euthanasia is the act of humanely killing animals by methods that induce rapid unconsciousness and death without pain or distress.¹
POLICIES AND GUIDELINES
“PHS Policy and USDA Regulations require that an IACUC review and approve the methods of euthanasia which are proposed. These must be consistent with the recommendations of the AVMA Guidelines on Euthanasia or succeeding revised editions, unless there are scientific justifications for alternative methods.” “… criteria used to evaluate the appropriateness of a given method include compatibility with the requirements of the research, reliability, irreversibility, the minimization of distress to animals and persons performing euthanasia, and safety to the latter. The species of animal being used and the qualifications of the investigators are also important considerations. Three categories of methods exist: inhalation and non-inhalation pharmacologic agents, and physical methods.” ²
- Inhalation Agents
· Carbon Dioxide (CO2)
Prolonged carbon dioxide inhalation is an effective and approved method of euthanizing small (less than 800 grams) rodents and small birds when it is done in accordance with the following guidelines. In fact, CO2 euthanasia has several advantages over other methods of euthanasia. For example, carbon dioxide is a potent central nervous system depressant and thus causes rapid unconsciousness and anesthesia. Carbon dioxide exposure has also been shown to induce analgesia that begins within a few minutes of exposure and lasts for as long as an hour. Carbon dioxide is a relatively inert, inexpensive and easily procured gas that is not very hazardous for exposed humans. Finally, carbon dioxide does not accumulate in or contaminate tissues and has minimal effects on tissue architecture (with the exception of the lungs). Nonetheless, since inhalation of carbon dioxide is known to cause mucosal irritation and thus may cause short-term stress in animals exposed to this gas, a few precautions are warranted:
a. The current AVMA guidelines (2020) recommend gradually filling of euthanasia chambers at a displacement rate of 30-70% of chamber volume per minute. This requires the use of correct equipment to deliver the gas at a pre-defined rate (e.g., an appropriate pressure-reducing regulator and a flow meter or equivalent equipment). The practice of placing animals in a prefilled container of 100% CO2 is unacceptable.
b. 100% CO2 from a compressed gas tank must be used. It is not acceptable to use CO2 from a dry ice source. The use of CO2/oxygen mixtures prolongs the time until unconsciousness and death and does not reliably eliminate signs of distress.
c. CO2 flow into the chamber should be maintained for at least one minute after animals have stopped breathing, and animals should remain in the chamber for at least one minute after the gas flow has stopped. Regardless of how long the animals are exposed to the CO2, a secondary physical method (e.g., thoracotomy, decapitation or vital organ removal) must be performed to ensure that the animal is dead.
Note: Rodents under ten days of age are very resistant to the effects of CO2 and require extended exposure times. Rapid decapitation is recommended for mice and rats under ten days of age. Mice and rats > 10 days of age may be treated as adults.
· Acceptable Inhalation Agents
Isoflurane is an acceptable agent with or without pre-medication. As with carbon dioxide, death of the animal must be ensured with a secondary physical method. It is important to minimize potential hazards to personnel by using a fume hood, down-draft table, or other scavenging device.
- Non-Inhalation Agents
· Acceptable Non-Inhalation Agents
Sodium pentobarbital is the most commonly used non-inhalation euthanasia agent. A correct dosage is critical; the euthanasia dose is typically three times the anesthesia dose. Pentobarbital may be delivered intraperitoneally or intravenously in rodents; in larger animals, the agent must be delivered intravenously (or intra-cardiac if the animal already is under anesthesia). A secondary physical method must be used to ensure death.
Overdose with ketamine/xylazine, tribromoethanol, urethane, or other chemical anesthesia agents is also conditionally acceptable at a dose two to three times the anesthetic dose.
· Unacceptable Non-inhalation Agents
Potassium chloride is unacceptable unless used under anesthesia.
- Physical Methods
Anesthesia must be used prior to the use of any physical method unless exceptions are approved with proper scientific justification and training.
Physical methods of euthanasia include cervical dislocation, decapitation, exsanguination, and pithing. However, some of these procedures, namely exsanguination, and pithing are not recommended as sole means of euthanasia.
· Cervical Dislocation
This method is primarily reserved for euthanasia of mice and neonatal rats. “Cervical dislocation is acceptable with conditions for mice and rats weighing <200g. Personnel should be trained on anesthetized and/or dead animals to demonstrate proficiency.”3 Training and documentation by the University Veterinarian in this technique is required.
· Decapitation
This method requires scientific justification when used without prior anesthesia. Physical hazard to the investigator must be taken into consideration and it is essential that the equipment be properly maintained with appropriate documentation. Training and documentation by the University Veterinarian in this technique is required.
Decapitation without anesthesia is conditionally acceptable in neonatal altricial rodents.
The American Veterinary Medical Association (AVMA) Guidelines on Euthanasia state that, "The equipment used to perform decapitation should be maintained in good working order and serviced on a regular basis to ensure sharpness of blades." In addition, the IACUC will review guillotine maintenance records during the campus’s semimonthly site visits.
· Physical Methods of Ensuring Euthanasia
Following chemical euthanasia, a physical method is required to ensure death. Physical methods include decapitation, thoracotomy; removal of a vital organ, cervical dislocation; and pithing (amphibians).
Footnote 1. Guide for the Care and Use of Laboratory Animals, 8th Edition, page 123
Footnote 2: Institutional Animal Care and Use Committee Guidebook, OLAW and ARENA, Section C.2.b.
Footnote 3: AVMA Guidelines on Euthanasia, 2020
https://www.avma.org/sites/default/files/2020-01/2020_Euthanasia_Final_1-15-20.pdf
15.7 Emergency Evacuations during Animal Surgery Policy
Biomedical research frequently involves surgical and other procedures in animals. If such procedures are underway when evacuation of the laboratory is required for safety reasons, (e.g. fire, gas leak, bomb threat, etc.), human safety must take precedence over potential loss of animal life. In such a situation, all personnel must leave the building immediately. However, it is not acceptable to leave animals unattended in the middle of a procedure. Therefore, unless the animals are able to recover from the procedure (e.g. awaken from anesthesia) safely without pain and distress and in a safe environment (e.g. returned to home cage), they must be euthanized immediately using approved methods prior to evacuation.
15.8 Policy for Care of Animals During an Emergency or Disaster
The University of Vermont has a comprehensive Emergency Response and Recovery Plan. The Office of Animal Care Management (OACM) has developed a supplement to the University’s Plan to further define what specific care of animals at the University is required in the event of an emergency.
In an emergency situation, animals will be cared for by OACM or other responsible personnel as described in these plans. The plans are on file in the animal facilities and farm offices, as well as with other appropriate University emergency personnel.
IACUC policy 15.8 Emergency Evacuations during Animal Surgery must be followed in the event of an emergency that requires evacuation of a laboratory or building.
15.9 Environmental Enrichment and Social Housing for Laboratory Animals
According to the Guide for the Care and Use of Laboratory Animals (National Academy Press, 8th ed, 2011), “the primary aim of environmental enrichment is to enhance animal well-being. This is accomplished by providing stimuli, structures, and resources that facilitate the expression of species-typical behaviors. Well-conceived enrichment provides animals with choices and a degree of control over their environment.” For social animals, the ability to interact with other animals provides a degree of enrichment; according to the Guide, “social animals should be housed in stable pairs or groups of compatible individuals unless they must be housed alone for experimental reasons or because of social incompatibility.”
At UVM, enrichment may include, but not be restricted to, the following items: nesting material such as nestlets or bedding sheets; nylabones or other gnawing media; opaque plastic or paper huts or tunnels; and running wheels.
- Animals routinely will be housed in solid-floor cages or pens with bedding unless specific scientific justification for grid or wire-mesh flooring is provided in the IACUC protocol by the investigator.
- Mice routinely will be given nesting media (e.g. nestlets) unless the investigator prefers another form of enrichment.
- Similarly, rats routinely will be given gnawing substrate (e.g. nylabone or wood).
- Any of the above mentioned enrichment items, or other items as suggested by the investigator, may be utilized.
- If NO enrichment item is to be provided, the absence of enrichment must be specifically justified in the IACUC protocol.
Similarly, social species must be housed in stable, compatible social groups whenever possible. If social housing is not possible for experimental reasons, those reasons should be stated in the IACUC protocol. Examples of justification for single housing include cannulae and other implants which may be disrupted by cage-mates, or the need to monitor individual feed or water intake. It is NOT necessary to justify single-housing of animals if it is done because of social incompatibility or veterinary-related concerns about animal well-being (e.g. post-surgical animals). However, individual-housing should be limited to the minimum period necessary, and additional enrichment should be provided for individually-housed animals.
15.10 Policy on euthanasia of horses
As a responsible steward of the UVM Morgan Horse Farm, the University of Vermont complies with the position of the American Association of Equine Practitioners on the euthanasia of animals. The University accepts that humane euthanasia of chronically ill or debilitated animals or horses deemed unfit for adoption is an acceptable procedure once available alternatives have been explored. A horse should not have to endure persistent pain, confinement or care erosive of the animal’s quality of life. This is in accord with the role of the University as an advocate for the animals under its care.
The following are guidelines to assist in making humane decisions regarding euthanasia of horses.
- A horse should not have to endure continuous or unmanageable pain from a condition that is chronic and incurable.
- A horse should not have to endure a medical or surgical treatment that, in the judgement of the Herd Veterinarian, has a poor to non-existent chance of success.
- A horse should not have to remain alive if it has an unmanageable medical condition that renders it a hazard to itself or its handlers.
- A horse should not have to receive continuous analgesic medication for the relief of pain for the rest of its life.
- A horse should not have to endure a lifetime of continuous individual box stall confinement for prevention or relief of unmanageable pain or suffering.
Decisions with regards to the euthanasia of horses at the Morgan Horse Farm are made by the Herd Manager in consultation with the Herd and/or University Veterinarian and the Chair of the Department of Animal & Veterinary Sciences. As horses are livestock animals with a definable economic value, the decision to treat or euthanize an animal should take into account the individual’s potential genetic and/or economic contribution to the herd. In the event of an urgent situation that precludes consultation with people who are not on-site, the Herd Manager is empowered to make the decision whether to treat or humanely euthanize an animal.
Any euthanasia which is performed at the Morgan Horse Farm shall be performed by a veterinarian in full compliance with the AVMA Guidelines for the Euthanasia of Animals. The Institutional Animal Care & Use Committee should be informed of any instances of death or euthanasia which occur on the Morgan Horse Farm.
15.11 Food or fluid restriction definitions, policies and guidelines
Food or Fluid Restriction Definitions
- Standard for Food Intake: “Animals should be fed palatable, uncontaminated diets that meet their nutritional and behavioral needs at least daily, or according to their particular requirements . . . “ p. 65 in the Guide for the Care and Use of Laboratory Animals, NRC 8 th ed. 2011 (the Guide). The IACUC interprets this to mean "ad lib" feeding unless otherwise justified.
- Standard for Water Intake: "Animals should have access to potable, uncontaminated drinking water according to their particular requirements." (P. 67, " the Guide , 2011). The IACUC interprets this to mean "ad lib" feeding unless otherwise justified.
- Restriction is any deviation from the standards for food and water intake.
- Deprivation is total withholding of either food or fluid.
- Fasting for surgical procedures is usually for a period of less than 12 hours, with the exception of farm animals which may require a longer period of fasting. This is not considered deprivation and the following guidelines below do not apply. Pre-operative fasting of rodents is not recommended except as specific procedures require.
Food or Fluid Restriction Policy and Guidelines
The IACUC endorses as policy the following excerpt from page 31 of the Guide:
“The development of animal protocols that involve the use of food or fluid regulation requires the evaluation of three factors: the necessary level of regulation, potential adverse consequences of regulation, and methods for assessing the health and well-being of the animals. . . . The animals should be closely monitored to ensure that food and fluid intake meets their nutritional needs. Body weights should be recorded at least weekly and more often for animals requiring greater restriction.”
Restriction of either food or fluid intake should be justified based on the scientific objectives of the study, and the least restriction that will achieve these objectives should be used. The plan for appropriate periodic weighing, starting with a pre-experimental weight, and monitoring of animal health must be included in the protocol. When food deprivation or restriction is planned, the following humane endpoints apply, unless alternate endpoints have been appropriately justified and approved by the veterinarian and the IACUC:
- Animals that acutely lose more than 20% of their body weight (compared to matched controls and/or a normal anticipated growth curve) should be eliminated from the study (euthanized) or placed back on a normal diet.
- When weight losses greater than 30% occur over a prolonged period of time (taking into account the normal anticipated growth for that animal), the deprivation or restriction must be terminated or the animal must be euthanized if weight loss continues beyond 30%.
This policy should not be construed to prohibit moderate caloric restriction of animals which are being housed for long-term studies. “Management of caloric intake is an accepted practice for long-term housing of some species . . . for example; there is good evidence that mice and rats with continuous access to food can become obese with attendant metabolic and cardiovascular changes. . . Benefits of moderate caloric restriction in some species may include increased longevity and reproduction, and decreased obesity, cancer rates and neurogenerative disorders. “(p. 67, the Guide). However, a plan should still be included in the protocol for periodic (at least weekly) monitoring of body weights for animals on caloric restriction.
15.12 Housing Animals Outside of and/or Removal of Animals From the Central Animal Facility
Definitions:
Study Area: “Study area is defined as any building room, area, enclosure or other containment outside of a core facility or centrally designated or managed area in which animals are housed for more than 12 hours”. 9CFR Chapter 1§ 1.1
Satellite Facility: “A satellite facility is any containment outside of a core facility or centrally designated or managed area in which animals are housed for more than 24 hours.” - Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Policy and Procedures:
It is the General Policy of the University of Vermont/Institutional Animal Care and Use Committee (IACUC) to centralize animal facilities as much as possible. Removal of animals outside of the Central Animal Facility and housing animals outside of the Central Animal Facility in a study area or satellite facility for more than 12 hours must be justified. The Guide allows for the use of special environments with proper justification. Therefore, all of the specifics of the Guide may not need to be addressed for every study area or satellite facility. However, each protocol must address the basic principles for proper care and use of animals as outlined in the Guide. Because a study area or satellite facility is outside the Central Animal Facility, it becomes the responsibility of the Principal Investigator to ensure that all of the specifics included in the protocol for care and use of the animals are carried out. It is also the PI’s responsibility to see that all personnel having incidental exposure to the animals are enrolled in the Occupational Health Program. Veterinary oversight remains the same as if the animals were in the Central Animal Facility and additional IACUC oversight may be required. (e.g., semi-annual inspections for Study Areas).
Policy on Housing Rodents in Laboratories
Vertebrate animals on the grounds of the University of Vermont will normally only be held for up to twelve hours outside standard animal holding facilities. Under specified and approved conditions animals may be housed for longer periods. Those specified conditions include the following:
- Prior inspection and approval of the spaces by an IACUC appointed site visit team.
- Rodents must be held in 100% exhausted air hoods certified by Environmental Health and safety, in semi-rigid isolators. Exhaust air hoods must be dedicated solely to animal holding.
- Numbers of rodents will be limited to those that will fit in the hood or the isolator within space recommendations for a given species.
- Posting of the emergency veterinary roster on call with phone numbers in the immediate area is required.
- In cases where animals are to be housed for >24 hours, if work cannot feasibly be completed in a hood or a hood is not available, animals can be housed in dedicated lab space as long as the space receives a minimum of 10 air changes per hour (must be tested annually by Physical Plant).
The hood or lab environment will comply with the same standards as animal rooms in the central animal facilities. This includes the following:
- Documentation of observation and care posted on a daily log near the hood with entries for every day animals are present. This daily documentation must include when cages are changed, maximum, minimum, and current temperature in the hood recorded, frequency, duration, how animals were manipulated and any problems noted with food and/or water intake. This information must be readily available to the University Veterinarian, IACUC members and Animal Care Management.
- Sanitization of the hood prior to housing rodents is required.
- Documentation of light cycles in the room (time lights turned on and off) needs to be included on the daily log sheet.
- Identification of the person responsible for the daily care of the animals being held in the hood, with emergency notification information.
- Problems are to be phoned into a clinical veterinary staff person at 802-656-8708 or refer to posting of emergency numbers.
- Every animal cage must be properly identified and include the following information: Investigator name and emergency phone number; Protocol number; Species and stock or strain; Source of the animal(s); Pertinent dates.
Laboratories with hoods holding animals will be site visited at least every six months by a team from the IACUC to evaluate standards of care and handling and to certify that the requirements of the “Guide” are being met. In addition representatives from the clinical veterinary staff of OACM will make periodic checks to evaluate animal health.
15.13 Post-Approval Monitoring of Protocols Policy and Procedures
Protocol Monitoring Policy
An integral part of UVM’s program for the care and use of animals is meaningful monitoring of protocols that have been approved by the IACUC. Each protocol submitted to the IACUC requires the Principal Investigator (PI) to sign an assurance that the protocol accurately reflects the procedures and care that affect research animals in any way. Post-approval monitoring is an ongoing process that ascertains that investigators are adhering to their protocol and documenting that adherence. According to the Guide for the Care and Use of Laboratory Animals (NRC, 2011), the IACUC may utilize a variety of opportunities to monitor a protocol, including but not restricted to: “continuing protocol review; laboratory inspections (conducted during regular facility inspections or separately); veterinary or IACUC observation of selected procedures; observation of animals by animal care, veterinary, and IACUC staff and members, and external regulatory inspections and assessments.” Post-approval monitoring should “support a culture of care focusing on the animals’ well-being.”
At UVM, the University Veterinarian is primarily responsible for the thoroughness and accuracy of post-approval monitoring. Compliance will be monitored during both scheduled and unscheduled visits to the animal and laboratory facilities. If assistance is required with this monitoring, the University Veterinarian may draw on OACM or RPO staff or members of the IACUC for help in reviewing animal and laboratory records.
The protocol follow-up and monitoring process will address the following areas:
- Protocol: up-to-date, agrees with procedures used by researchers
- Personnel: listed in the protocol, verify adequacy of training by records or observation of procedures
- Records: animals identified, activities documented, proper surgery & anesthesia records, animal observation documented, unscheduled deaths or other health problems documented and veterinarians notified
- Laboratory: locations stated correctly in protocol, lab maintained in proper condition, correct storage of pharmaceuticals and other chemicals; substances used in animals are in-date.
Laboratory personnel will be notified to correct any noncompliant areas identified in the process. The PI and the IACUC will receive a written report of the visit. It is the responsibility of the PI to correct deficiencies in a timely manner. It is the responsibility of the IACUC to confirm with the PI that corrections have been made and adequately documented.
15.14 Policy on Experimental Neoplasia in Rodents
Purpose
The purpose of this document is to provide guidelines for the UVM research community on experimentally induced neoplasia in rodents. Investigators producing tumors in rodents should use this document as a reference in preparing their Animal Care and Use Protocols. These guidelines may be reviewed and followed as written, or exceptions may be requested.
Policy
Legal and regulatory guidelines require that animal pain, distress, and suffering be minimized in any experiment. In order to minimize pain and distress in studies on experimental neoplasia in rodents, investigators must address issues concerning tumor burden, the status of the tumor (e.g. ulceration), and criteria for euthanasia. The following guidelines are intended to provide investigators with an indication of the standards and criteria for euthanasia that have been considered acceptable by the IACUC. The IACUC will evaluate an investigator’s request for an exception to these policies. The scientific rationale for the exception must be clearly stated.
Tumor Burden
In general, tumor burdens should not be so large as to interfere with ambulation, eating, drinking, defecating, and urinating. The visible size of the tumor is only one of the criteria used for determination of humane endpoint. The overriding consideration for humane endpoints of oncological experiments as well as spontaneous tumors must be the overall health of the animal. For subcutaneous tumors, the maximum allowable size is 20 mm in diameter for a mouse or 40 mm diameter for a rat. If the animal is host to more than one tumor, this size is the maximum allowable size for all tumors combined. Without a specific exception justified in the animal protocol, animals should be euthanized before tumors reach this size.
Tumor Status
Ulceration is often an inevitable complication of subcutaneous tumors. Animals with ulcerations or necrotic tumors must be euthanized unless an exception has been approved.
Monitoring of animals with tumors
In addition to the daily health checks performed by the animal care staff, documented daily health checks should be initiated by the investigator before the tumor begins to interfere with the physiological function of the animals.
Criteria for Euthanasia
Investigators must administer euthanasia in moribund animals. The IACUC requires that an investigator judge when euthanasia is appropriate for moribund rodents based on objective signs. Some of the known signs of illness or impending death which may be applied are listed below.
- Impaired mobility (the inability to reach food and water)
- Inability to remain upright
- Significant weight loss
- Significant abdominal distension, especially when it begins to compromise respiratory ability of animal,
- Hunched posture with easily visible vertebral bodies
- Failure to eat or drink
- Absence (or abnormal) fecal or urine output
- Rough hair coat
- Reluctance to move or abnormal gait
- Discharges or hemorrhage
- Abnormal behavior or vocalizations
15.15 Determination of levels of pain & distress definitions, policies and guidelines
USDA Pain Levels:
Level B: Breeding animals or Holding Protocol animals; no procedures performed.
Level C: No more than momentary or slight pain or distress. For example: euthanasia; observation under non-stressful conditions; positive reward behavioral tests.
Level D: Pain or distress relieved with anesthetics, analgesics and/or tranquilizer drugs or other methods for relieving pain or distress. For example: survival surgery, non-survival surgery, induced infections or antibody production with appropriate anesthesia and post-op/post-procedure analgesia when necessary.
Level E: Pain or distress or potential pain or distress that is not relieved with anesthetics, analgesics and/or tranquilizer drugs or other methods for relieving pain or distress.
According to the Guide (p27-28), Level E studies require special consideration during IACUC review. “The IACUC is obliged to weigh the objectives of the study against potential animal welfare concerns.” The appropriate use of humane endpoints should be carefully considered during experimental planning and IACUC review.
(Note, there is no USDA level A.)
- Euthanasia - Euthanasia is the act of inducing humane death in an animal. Euthanasia techniques must result in rapid unconsciousness followed by cardiac and respiratory arrest and ultimate loss of brain function. In addition, the technique should minimize any stress and anxiety experienced by the animal prior to unconsciousness. Stress may be minimized by technical proficiency and humane handling of the animals to be euthanized. For guidance, see 2020-Euthanasia-Final-1-17-20.pdf (avma.org) (2020 Edition)
- Non-Survival Surgery includes any surgical procedure where there is the potential for more than momentary or slight pain prior to death, including those due to procedures requiring extended time periods. Any protocol that includes surgical procedures not directly associated with euthanasia and occurring prior to euthanasia should be categorized as non-survival surgery.
Guidelines for determining USDA classification in protocols involving tissue collection before/after euthanasia and/or animal perfusion:
- If an animal will be euthanized by an approved physical or chemical method of euthanasia solely for the collection of tissues (after the animal's death), the procedure should be classified as USDA C.
- If an animal will be anesthetized so that non-vital tissues can be collected (liver or skin biopsy), and the animal will then be allowed to recover, the procedure should be classified as USDA D (survival surgery).
- If an animal will be anesthetized so that non-vital tissues can be collected (liver or skin biopsy, etc.), and the animal will then be euthanized, the procedure should be classified as USDA D (non-survival surgery). In this scenario, it is necessary to justify why the animal could not be euthanized (USDA category C) rather than anesthetized.
- If an animal will be anesthetized so that vital tissues can be collected (heart, both kidneys or lungs, whole liver, etc.), the animal will obviously succumb to the procedure. To determine whether this will be euthanasia or non-survival surgery, we must consider the definition of euthanasia. A critical component of this definition is "rapid unconsciousness followed by loss of cardiac, respiratory and brain function". Based on this definition, procedures that require tissue manipulation or other prolonged (more than a few minutes) techniques prior to the animals death should be classified as non-survival surgery (USDA D). Similarly, if an animal will be anesthetized so that the tissue can be collected in the "freshest" possible state (i.e., heart) and the tissues will be rapidly excised, the procedure should be classified as euthanasia (USDA C). (Note: In this scenario, it is difficult to justify why the animal couldn't be euthanized rather than anesthetized.)
- If an animal will be anesthetized so that it can be chemically perfused, the same "test of time" applies (i.e., long, technical manipulations should be classified as USDA D, while rapid intravascular injection of the perfusate without other manipulations should be classified as USDA C).
- Agricultural animals used in teaching protocols (e.g. cows, horses) should be classified as pain level “C,” as a number of routine activities (e.g. blood sampling, injections of vaccines or pharmacologic agents) involve only momentary pain. Any agricultural animal undergoing a procedure that involves more than momentary pain/distress, such as dehorning or the surgical correction of a displaced abomasum, must receive intra- and post-procedural analgesia as appropriate to the invasiveness of the procedure and should be classified as pain level D.
NOTE: Because the USDA classification system is based on the "potential for pain, distress or discomfort," the anesthetic/euthanasia drug dose becomes a critical concern. For example, if a known "euthanasia dose" of pentobarbital will be administered, drug irreversibility is assumed. Thus, once the animal is confirmed to be in an anesthetic plane (lack of toe pinch response, etc.), tissues can be collected/ procedures can be performed without the concern about what the animal is perceiving. This procedure would then be classified as USDA C. The Committee recommends using a euthanizing dose whenever possible. Other methods may be appropriate with proper scientific justification.
15.16 Physical restraint - definitions, policies and guidelines
Physical Restraint Definitions
- "Physical restraint is the use of manual or mechanical means to limit some or all of an animal’s normal movement for the purpose of examination, collection of samples, drug administration, therapy, or experimental manipulation" (p. 29, "Guide for the Care and Use of Laboratory Animals," 8th ed., National Research Council, 2011).
- Prolonged restraint includes any procedure involving restraint for a duration of time which could lead to distress. Distress is present when the animal is unable to compensate in response to a stressor.
- Although the definition of prolonged restraint may vary depending on the species of animal and the type of restraint, the IACUC at the University of Vermont considers any restraint that prevents an animal from making normal postural adjustments for a period of greater than 30 minutes to be prolonged.
Physical Restraint Policy and Guidelines
According to the Guide (p29-30), “Prolonged restraint should be avoided unless it is essential for achieving research objectives and is specifically approved by the IACUC . . . The period of restraint should be the minimum required to accomplish the research objectives.” “When restraint devices are used, they should be specifically designed to accomplish research goals that are impossible or impractical to accomplish by other means or to prevent injury to animals or personnel.”
According to the American Society of Laboratory Animal Practitioners, “Habituation (acclimation) of the animal to the restraint device should be considered when the animal may be repeatedly restrained over the course of a study or medical treatment, or restrained for an extended period of time.”
A protocol that utilizes prolonged restraint must include a plan for appropriate monitoring of animals throughout the entire period of restraint and a description of how stress and/or distress will be evaluated. If unanticipated distress is encountered with use of any physical restraint, it should be promptly reported to the veterinary staff.
- Restraint devices and experimental equipment should be suitable in size, design, and operation to minimize discomfort, pain, distress and the potential for injury to the animal and the research staff.
- Restraint devices and experimental equipment are not considered normal methods of housing.
- Restraint devices and experimental equipment should not be used simply as a convenience in handling or managing animals.
- Veterinary care should be provided if lesions or illnesses associated with restraint are observed. The presence of lesions, illness, or severe behavioral change often necessitates temporary or permanent removal of the animal from restraint or experimental equipment.
- The purpose of the restraint and use of experimental equipment and its duration should be clearly explained in the IACUC protocol and to the personnel involved with the study.
- Animals must be habituated to restraint devices and experimental equipment prior to beginning an experimental series unless justified in your IACUC protocol
15.17 Reporting Animal Deaths
At the termination of the research study, all animals must be assigned to one of three categories in the Animal Care and Use Protocol:
1. The animals will survive the study unharmed, and then be available either for redistribution or adoption (both of which must be coordinated through the Office of Animal Care Management).
2. The animals will be euthanized.
3. The animals will die prior to euthanasia due to the experimental design. This is termed "death as an endpoint" and requires special justification to be approved by the IACUC.
Occasionally, however, an animal falls into a fourth category and dies UNEXPECTEDLY prior to collection of useable data. The cause of death may or may not be known Federal regulations require that animal deaths be tracked and reviewed by the Veterinarian and IACUC. This is also important for the animal program at the University because unexpected animal deaths may be an indicator either of health problems in the colony or procedural problems in the experimental model.
Deaths that must be reported
1. Animals that are found dead by a caretaker, technician, investigator, veterinarian, etc. must be reported even if you expected the animal to die and this was stated in the Animal Care and Use Protocol. If a necropsy by the veterinary staff is requested, please bag, tag, and place the carcass in a designated refrigerator (please do not freeze).
2. Animals that must be euthanized before they can yield usable data for the study.
3. An unscheduled death in a USDA-covered animal species must be reported directly to the veterinarian or a veterinary technician.
How to report animals
Complete the Animal Necropsy Form (available from the Office of Animal Care Management). When completing this form, be sure to do the following:
1. Provide all the information requested on the upper portion of the report.
Describe any relevant history (e.g. recent surgical or drug manipulations) in the space for “clinical history.”
2. Indicate if the animal was euthanized or found dead prior to data collection in the appropriate places on the form.
3. If the death was expected and the protocol specifically addresses the issue of "Death as an endpoint", mark the box that says "Experimental" on the form.
The carcass of the animal should be placed in a small black plastic bag (provided in all animal holding rooms) and then be placed in the facility’s dedicated carcass freezer (C024 Given; HSRF 005I; T216 CRF; 428 JDH).
Tracking animal deaths
All completed Necropsy Forms will be submitted to the Office of Animal Care Management. The Veterinary Technicians screen the forms and contact the Veterinarian if veterinary assistance is required (e.g. necropsy, lab work). The data from the necropsy form will be entered into the appropriate database. Necropsy, lab findings, diagnoses, etc. will be reported (by verbal and/or written form) to the appropriate individual(s) (usually the investigator that submits the request). A monthly report summarizing animal deaths will be submitted to the IACUC by the Office of Animal Care Management.
15.18 Rodent Cage Density
Overcrowded mouse cages represent a significant animal welfare concern. Such cages are noncompliant with Public Health Service (PHS) Policy and our Assurance to PHS. The Guide for the Care and Use of Laboratory Animals states the PHS recommendations for housing densities. In order to standardize housing densities and prevent or eliminate the possibility of overcrowding within cages, the Office of Animal Care Management has adopted the following IACUC-approved policy. Cage sizes and animal numbers permitted are listed in the table below. Cage densities exceeding these numbers represent clear policy violation.
Table of Maximum Rodent Cage Densities
| Mouse | Super Mouse™ 750 Cage | Super Mouse™ 1800 Cage |
|---|---|---|
| < 10 g | 12 Mice | 30 mice |
| 10-15 g | 9 Mice | 22 mice |
| 15-25 g | 6 Mice | 15 mice |
| > 25 g | 5 Mice | 12 mice |
| Female & litter | One plus one additional adult | Three plus one additional adult |
Table of Maximum Rodent Cage Densities
| Rat | One Cage™ Rat | One Cage™ Rat 2100 |
|---|---|---|
| < 100 g | 5 rats | 12 rats |
| 100-200 g | 4 rats | 8 rats |
| 200- 300 g | 3 rats | 7 rats |
| 300 -400 g | 2 rats | 5 rats |
| 400-500 g | 1 rat | 3 rats |
| > 500 g | 1 rat | 2 rats |
| Female & litter | Not permitted | One |
Floor Area for Ventilated Cages
- Super Mouse™ 750 = 75 sq in
- Super Mouse™1800 = 180 sq in
- One Cage™ Rat = 90 sq in
- One Cage™ 2100 Rat = 210 sq in
Overcrowded Cages
Overcrowded cages will be reported to investigator. OACM will remove mice from overcrowded cages if the investigator has not done so within 24 hours of notification. There is a fee for this service.
Identification
A completed and legible cage card must be present on all rodent cages. The information on the card should include: the investigator's name and current contact information for the PI or a responsible lab member, the approved IACUC protocol number, the animal source and date of birth or arrival at the facility, the animal's species and strain or stock. Individual animal identification must follow the Animal Identification Policy and be described in the animal protocol and approved by the IACUC.
The OACM staff is available to discuss any questions you may have regarding this policy.
15.19 Procurement and Storage of Controlled Drugs - Policy & Procedures
Controlled substances (CS) are often used in research settings for anesthesia, analgesia, sedation or euthanasia. Controlled substances are drugs which are regulated by the Drug Enforcement Administration (DEA) because of potential for abuse.
Each investigator or research location which uses CS must complete a registration with the DEA and obtain approval from the UVM Controlled Substances Committee (CSC) following the University Operating Policy – Controlled Substances in Research.
15.20 Surgery - definitions, policies and guidelines
IACUC accepts the following definitions from the 8th edition of the "Guide for the Care and Use of Laboratory Animals." National Academies Press, 2013.
“As a general guideline, major survival surgery (e.g., laparotomy, thoracotomy, joint replacement, and limb amputation) penetrates and exposes a body cavity, produces substantial impairment of physical or physiologic function, or involves substantial tissue dissection or transection . . . Minor survival surgery does not expose a body cavity and causes little or no physical impairment . . . When attempting to categorize a particular surgical procedure, the following should be considered: the potential for pain and other postoperative complications; the nature of the procedure as well as the size and location of the incision(s); the durations of the procedure; and the species, health status, and age of the animal. . . In non-survival surgery, an animal is euthanized before recovery from anesthesia.” (Guide, p. 117-118)
“Multiple surgical procedures on a single animal should be evaluated to determine their impact on the animal’s well-being. Multiple major surgical procedures on a single animal are acceptable only if they are 1) included in and essential components of a single research project or protocol, 2) scientifically justified by the investigator or 3) necessary for clinical reasons. Conservation of scarce animal resources may justify the conduct of multiple major surgeries on a single animal, but the application of such a practice on a single animal used in separate protocols is discouraged and should be reviewed critically by the IACUC. If multiple major survival surgery is approved, the IACUC should pay particular attention to animal well-being through continuing evaluation of outcomes. Cost savings alone is not an adequate reason for performing multiple major survival surgical procedures.” (Guide, p.30)
In addition, the protocol must have follow-up observation beyond what is required for standard single survival surgeries. This observation should include an awareness of the second surgery during the pre-surgical, the surgery and the post-surgical periods. Follow-up should also include any special procedures or extra care required during the period between surgeries.
It is IACUC policy that all surgical facilities need to be inspected by IACUC prior to their use.
SURVIVAL SURGERY
- Pre-Surgical Preparation for Single or Multiple Survival Surgeries:
Procedures must be outlined in the protocol for preparation of the animal for surgery. These procedures must include aseptic preparation of the surgical site including shaving of fur and appropriate disinfection of the skin, pre-surgical medication(s), and food or fluid restriction (if applicable). Procedures must minimally include the use of sterile instruments (see below for instrument sterilization techniques), surgical masks and gloves, surgical draping as appropriate, and aseptic preparation of the surgery site.
- Minor and Rodent Surgery
Survival surgical procedures which do not qualify as “major” and surgeries performed on mice of the genus Mus and rats of the genus Rattus do not require a dedicated surgery facility. However, the area of the laboratory or room where surgery is performed should not be used for other functions at the time that surgery is in progress and the area should be clean and free of clutter.
- Major Surgical Procedures on Species other than Mice and Rats
Major survival surgical procedures in mammals other than mice and rats must be conducted in a facility specifically intended and used only for that purpose and which is maintained and operated to ensure cleanliness. Additionally, a sterile surgical gown must be worn.
- Use of Paralytic Agents
Neuromuscular blocking agents, or “paralytics,” may be used as part of a regimen of balanced anesthesia to promote research objectives. However, it is the researcher’s responsibility to ensure that animals are not subjected to pain during these procedures. Because paralytic agents prevent an animal from responding to painful stimuli, monitoring methods that do not rely on animal movement must be used to ensure that an adequate plane of anesthesia is maintained. Examples of these methods include electrocardiography or pulse-oximetry.
- Post-Surgical Period
The post-surgical period is generally considered to be the period from the end of surgery to the point when the surgical wound is healed (e.g., time of suture removal). The period may be extended in cases where a physical impairment has been induced despite healed surgical wounds. During this time period daily recorded observations are required by qualified research team personnel.
NON-SURVIVAL SURGERY
Regardless of whether surgeries are survival or non-survival, animals must be maintained at an anesthetic plane that precludes pain and distress and recovery from anesthesia until the time of euthanasia. Although it may not be necessary to adhere to principles of aseptic technique for non-survival procedures, according to the Guide, “. . . At a minimum, the surgical site should be clipped, the surgeon should wear gloves, and the instruments and surrounding area should be clean." (Guide, p. 118)
INSTRUMENT STERILIZATION:
All instruments that come into direct contact with the surgical area must be sterile. Sterilization of instruments in surgical packs can be achieved in a number of ways:
- Steam Sterilization
- Instruments must be wrapped prior to autoclaving in either a disposable plastic autoclave pouch or autoclave wrap.
- Verification of autoclave function includes the use of indicators (e.g., autoclave tape, autoclave indicator strips inserted into the pack) to ensure the appropriate temperature has been reached during the cycle.
- Ethylene oxide
Surgical packs must be dated and used within six months of sterilization.
If surgeries are to be performed on consecutive animals, dry bead sterilization or chemical sterilant may be used. Sterilized instruments must be maintained on an aseptic field and instrument tips sterilized between animals. If using one of these methods, a new surgical pack of steam or gas sterilized instruments must be used after every 5 animals.
- Dry bead sterilizer; must have a minimum of one minute contact time:
- Remove organic material from the instrument before placing instruments in the bead sterilizer
- The instrument must be cooled prior to use on the animal
- Glass beads should be maintained according to manufacturer’s specifications and should be free of debris.
- Chemical sterilants: must have a minimum 10 minutes contact time. Isopropyl alcohol is not an appropriate cold sterilant; solutions such as dilute chlorhexidine, glutaraldehyde or MB-10/VIMOBA are appropriate
15.21 Testing of Biological Materials
Cell cultures and other biological products can be infected with a variety of murine viral pathogens. These pathogens constitute a risk to the biosecurity of UVM research animal colonies and potentially to human personnel. In addition, undetected viral contamination of cell lines may alter research results and negate the value of animal studies.
In order to minimize the risk of introducing murine pathogens into the research animal population, investigators who inject biological agents, such as cell lines, hybridomas or tumor cells, or products originating from these cell cultures, must submit those agents or products for testing by an approved diagnostic laboratory. A copy of the test result assuring that product is free of the relevant murine pathogens must be submitted to the Office of Animal Care Management prior to injecting the product into animals. For cell lines which are obtained from a vendor or a collaborator, a copy of test results assuring pathogen-free status may be submitted in lieu of a new test and will be reviewed by the University Veterinarian.
For murine-origin lines, the following laboratories and tests are acceptable:
- RADIL (University of Missouri), IMPACT test
- Taconic Laboratories (Frederick, MD), RapidMAP-21 test
- Charles River Laboratories (Wilmington, MA), Mouse Essential PCR Panel
Individual laboratories are responsible for the cost and arrangement of testing.
Biological materials of human origin
Any research conducted using animals that involve human materials and cell lines require handling at the ABSL-2 containment level and must be described in your IACUC protocol. Testing cell lines of human origin for human pathogens is not required unless you are seeking approval of a change in containment practices. See IBC Policy 3.5.2 Policy on Research Involving Human Materials and Cell Lines.
15.22 Animal Identification Methods
The regulations contained in the Guide for the Care and Use of Laboratory Animals stress the importance of proper animal identification in sound research and humane animal care. The Guide, as well as a number of other sources, lists many acceptable identification methods for most common laboratory animal species. A primary means of identification for rodents should be complete and accurate cage cards. These cards should include, at a minimum, the source of the animal, full strain or stock name, names and contact information for the responsible investigator(s), pertinent dates (e.g. arrival date, birth date, etc.) and protocol number. Animal Care Management provides printed cage cards for animals at the time of delivery from vendors or transfer from another protocol. If the investigator wishes to print cage cards, they are responsible for the accuracy of the above information. The number of animals per cage and genotype information, when available, should also be included. All breeder cages should be clearly identified.
The following are examples of acceptable methods for animal identification and must be described within the IACUC protocol:
- Collars, bands or plates (usually for larger animal species)
- Colored non-toxic ink markings on hair or skin
- Ear punches, notches or tags
- Microchips or subcutaneous transponders
- Tattooing (intradermal injection of non-toxic inks)
- Freeze branding (for amphibians)
Toe clipping should only be used when no other individual identification method is feasible. It may be the preferred method for neonatal mice up to 7 days of age as it appears to have few adverse effects on behavior and well-being at this age. If using toe clipping for the purposes of animal identification, it can only be performed when combined with genotyping and the PI must justify why other methods of identification are not feasible. Please refer to the Guide for other considerations related to this procedure if you propose to combine toe clipping and genotyping in your animal protocol..
15.23 Use of Expired Medical Materials Policy and Guidelines
Background
According to USDA Animal Care Resource Guide Policy on the use of expired medical materials (Policy #3):
The use of expired medical materials (e.g., drugs, fluids, sutures, anesthetics, sedatives, or analgesics) during any survival surgical procedure on a regulated species is not considered acceptable veterinary practice and therefore not consistent with adequate veterinary care as required by the regulations promulgated under the Animal Welfare Act.
Research, Teaching, and Testing Acute Terminal Procedures:
Expired medical materials except analgesics, sedatives, anesthetics, and euthanasia solutions may be used in acute terminal procedures where an animal is anesthetized during the study and euthanized without recovery if such use does not adversely affect the animal’s well-being or compromise the validity of the scientific study.
Facilities permitting the use of expired medical materials in acute terminal procedures should have a policy on the use, storage, and disposal of such materials which is in accordance with all relevant institutional, local, state, and federal requirements where applicable; and/or require investigators to describe the intended use in the animal study proposal.
The following guidelines concerning the use of expired medical material, developed by the Office of Animal Care Management and the IACUC at the University of Vermont, are based on the above USDA policy.
Expiration Dates:
The expiration date is the date printed on the label or package for materials with a manufacturer’s expiration. Secondary containers that hold a drug or material from an original stock to which no drug has been added must be clearly labeled with the name of the drug or material and the expiration date of the original stock. For dilutions, preparations, reconstitutions, or mixtures of drugs or fluids prepared using aseptic technique and under proper storage conditions, the expiration date is no more than 28 days from the date of preparation. Such materials must be labeled with name, drug concentration, and the new expiration date as soon as they are prepared. An item is considered expired the day after the month or date indicated on the label (i.e., an item labeled January 2020 is considered expired on February 1, 2020).
Powdered forms of drugs or compounds (e.g., chemical grade substances ordered from Sigma) that do not list an expiration date must be labeled with an expiration date of one year from the date received at UVM provided they are stored aseptically in an airtight, light-proof protective container. For drugs or solutions that are reconstituted for use, the expiration date may vary from the labeled expiration date. Reconstituted drugs and compounds that do not contain expiration or efficacy guidance in the directions must be labeled for expiration 28 days after reconstitution in a glass container.
When investigators wish to access sterile diluents without a manufacturer expiration date multiple times (e.g., to obtain small volumes for administration and drug mixing), the investigators may only do so if they do not add any chemical to the fluid, they access the fluid(s) aseptically, and they store the fluid(s) as recommended by the manufacturer. Under these conditions, the investigator may use the sterile fluid(s) for up to 28 days after initial opening.
Multi-dose containers, IV fluid bags or vials of liquid formulations
United States Pharmacopeia (USP) General Chapter 797 says the following regarding multi-dose vials of sterile commercial pharmaceuticals containing preservative:
- If a multi-dose container has been opened or accessed (e.g., needle-punctured) the vial should be dated and discarded within 28 days unless the manufacturer specifies a different (shorter or longer) date for that opened vial.
- If a multi-dose vial has not been opened or accessed (e.g., needle-punctured), it should be discarded according to the manufacturer’s expiration date.
- Preservative free containers or vials of a liquid formulation are typically considered single use, to be administered and discarded within 24 hours of opening/puncture of septum.
- Never leave a needle in the septum of a medication vial for multiple medication draws. This provides a direct route for microorganisms to enter the vial and contaminate the flui
Guidelines
FOR SURVIVAL PROCEDURES:
No expired drugs or materials may be used in survival surgeries.
Investigators are responsible for ensuring that all drugs and medical materials used in their laboratories are within the expiration date. All expired supplies must be labeled “Expired-Do Not Use” and stored separately from non-expired materials if immediate disposal is not possible.
FOR NON-SURVIVAL PROCEDURES:
With the exception of controlled substances and emergency, anesthetic, analgesic, or euthanasia drugs, expired medical materials may be used in terminal procedures, including non-survival surgeries, provided:
1. The use of expired medical materials is explicitly stated in the IACUC protocol and approval to use such materials has been granted by the IACUC. Items #4 and #5 (below) must be addressed in the IACUC protocol.
2. Materials are marked as "Expired – Use ONLY in TERMINAL Procedures."
3. Materials are stored in a different location (cabinet, drawer, etc.) than materials used for survival procedures.
4. The use of expired medical materials does not adversely affect the animal’s well-being or compromise the validity of the scientific study.
5. Proper anesthesia, analgesia, and euthanasia are employed for all such procedures.
IDENTIFICATION AND REMOVAL OF EXPIRED MEDICAL MATERIALS:
The IACUC recommends that each laboratory establish a procedure to facilitate the identification and removal of expired drugs and other medical materials used for research involving animals. This may take the form of a drug log, signed by a member of the laboratory each month, indicating that they have checked for and discarded, or set aside for disposal, any expired drugs or other medical materials from their laboratory. The IACUC reserves the right to make this recommendation a requirement if expired drugs or other medical materials are repeatedly identified in a particular laboratory.
Pharmaceutical agents should be disposed of in a safe and responsible manner. Drugs (other than simple solutions of saline or dextrose) may not be dumped down the drain. For guidance in disposing of drugs and other chemicals, consult with Environmental Health and Safety personnel. Some standard guidelines are available here:
https://www.uvm.edu/riskmanagement/laboratory-chemical-waste-management
All expired controlled substances must be disposed of according the Controlled Substances in Research University Operation Procedure.
15.24 Policy on Visitors
Visitors to the University of Vermont (UVM) who will work with, or be exposed to, animals must assure the IACUC that they have the necessary expertise regarding the proposed procedures and that they are either enrolled in an Occupational Health Program or are willing to waive that requirement. Enrollment in the Occupational Health Program at UVM is not an option for visitors. In addition, if visitors will perform procedures that potentially cause more than momentary pain or distress (e.g. survival or non-survival surgery), the Attending Veterinarian or a member of the veterinary staff must observe initial procedures to assure that the visitor adheres to UVM standards for those activities.
Before visitors can work with animals or be allowed access to the animal facility, the following steps must be accomplished:
- Principal Investigator must provide to the visitor a copy of the approved protocol for review,
- Principal Investigator must also provide a copy of the Visitor’s Certification for Waiver of Animal Care Training and Occupational Health Clearance Review to the visitor for completion (to be obtained from the IACUC office),
- Principal Investigator must collect the completed form from the visitor, sign it, and send it to the Veterinarian.
15.25 Rodent Breeding and Weaning Policy
Breeding
The three breeding schemes permitted are:
- Monogamous pairing (1 male: 1 female) - this method is preferred to prevent overcrowding.
- Trio grouping (1 male: 2 females) - females must be placed in individual cages prior to parturition.
- “Harem” breeding: (1 male: up to four females) not to exceed maximum number of adults permitted per cage.
In trio or harem breeding situations, male and female mice should be separated after visual confirmation of pregnancy (usually possible around 14 days of gestation) to avoid post-partum insemination. A postpartum estrus occurs within 14 to 28 hours after parturition in mice.
Female rats MUST be examined for pregnancy and separated from other females at 14 to 16 days of gestation.
No more than two adult mice (including the male) and one litter of pups may be housed in a standard mouse cage without IACUC approval for an exception to the recommendations of the Guide.
The breeding strategy must be described in the IACUC protocol. This includes the breeding scheme, whether continuous or non-continuous breeding will occur and the weaning age of pups. Justification is required for any scheme other than monogamous and trio or for cage densities which exceed those described above.
Weaning
Investigators who choose to manage their own breeding colonies are responsible for timely weaning. Mice are typically weaned at 21 days of age. At this age, the pups are placed on the animal census by the Animal Care staff. All litters must be weaned by 24 days of age unless otherwise justified in the animal protocol and approved by IACUC. Delayed weaning protocols must be approved by IACUC with specification of actual weaning ages.
- Investigators will be notified by phone or email if litters have not been weaned at 24 days of age.
- Twenty-four hours after notification, these mice will be weaned by OACM staff.
- There is a fee for this service.
Where continuous breeding is used, weaning of older litters between 19 and 21 days may be necessary if a second litter is born.
- Should the presence of an older litter constitute a threat to a newborn litter, OACM staff will notify the PI to separate within 24 hours.
- In the absence of a timely response by the investigator, OACM will wean the older litter for a fee.
- The investigator will be informed. The OACM staff provides training in the management of rodent colonies for investigators and their staff. OACM also offers colony management services to those PIs who choose this option.
15.26 Policy Regarding Aged Animals
This policy was inactivated on 5/24/2021
15.27 Policy Regarding the Use of Random-Source Animals for Research at the University of Vermont
The University of Vermont recognizes that the use of random-source animals for research purposes poses unique challenges to the Institutional Care and Use Committee (IACUC). This is particularly true when the species utilized are those which society traditionally regards as companion animals, such as dogs and cats.
Random-source animals are those which are procured from animal pounds or shelters, auctions, or any person who did not breed or raise the animals on his or her premises. Non-random source is a term used to describe animals which are bred and raised on the premises of an individual, such as a hobby breeder, although the animals are not purpose-bred for research and testing. A United States Department of Agriculture (USDA) Class B licensed dealer may procure animals from random or non-random sources, while a USDA Class A licensed dealer produces purpose-bred animals on his/her own premises which are subsequently sold for research or testing purposes.
Because the genetic and medical history of random-source animals is largely unknown, potential health and animal welfare problems may be associated with their use. It may be difficult for the IACUC to ascertain that animals have been reared, housed and transported under appropriate humane conditions. In addition, random-source animals may contribute unanticipated variation in scientific results due to their varied breed, medical status and prior standards of care.
Because of the potential animal welfare and scientific concerns associated with random source animals or animals procured from USDA Class B licensed dealers, the IACUC at the University of Vermont requires that investigators procure purpose-bred animals only from USDA Class A licensed dealers. This policy shall not apply to pigs used in biomedical research, agricultural animals, to amphibians, or to wildlife species used in field studies.
Reference: Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. National Research Council (US) Committee on Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Washington (DC): National Academies Press (US); 2009. Available at NSBI
15.28 Policy Regarding the Use of Non-Pharmaceutical-Grade Compounds in Living Animals
A pharmaceutical-grade compound is defined as any active or inactive drug, biologic or reagent, for which a chemical purity standard has been established by a recognized national or regional pharmacopeia [e.g., the U.S. Pharmacopeia (USP), British Pharmacopeia (BP), National Formulary (NF), European Pharmacopoeia (EP), Japanese Pharmacopeia (JP), etc.]. These standards are used by manufacturers to help ensure the products are of the appropriate chemical purity and quality, in the appropriate solution or compound, to ensure stability, safety, and efficacy.
The use of non-pharmaceutical-grade compounds often is necessary to accomplish the scientific aims of a research project. In these instances, the IACUC must consider the health and well-being of the animals while aiding the researcher in minimizing potentially confounding experimental variables and maximizing reproducibility of the research. The IACUC distinguishes between two scenarios when considering the use of non-pharmaceutical-grade compounds:
Clinical Use - compounds used for the clinical treatment of animals and to prevent or reduce/eliminate animal pain or distress. Whenever possible, pharmaceutical-grade compounds must be used for anesthesia* and analgesia.
Research Use - compounds used to accomplish the scientific aims of the study. If available, and suitable, pharmaceutical-grade compounds are preferred; but when non-pharmaceutical-grade preparations are used, the IACUC and the investigator must consider such factors as purity, sterility, vehicle, stability, pH, osmolality, site and route of administration, potential for adverse effects, and storage.
The IACUC must review and approve the use of non-pharmaceutical grade compounds on a case-by-case basis. The investigator is responsible for describing the use of the compound in sufficient detail that the IACUC can evaluate the potential for pain and/or distress in the research animals on which the product is being utilized. The investigator also is responsible for reporting adverse outcomes that arise from the use of non-pharmaceutical grade products.
* Pentobarbital euthanasia solutions use for anesthesia:
The administration of non-USP grade pentobarbital euthanasia solutions (e.g., Fatal+, Beuthanasia, Euthasol) for anesthesia is prohibited. Euthanasia solutions often contain other ingredients, such as muscle relaxants and dyes, and because they are non-USP, there is no assurance of chemical purity, quality, stability, or safety. Such proposed use will not be approved by UVM’s IACUC.
15.29 Policy on Re-use of Animals
The re-use of teaching or research animals that have previously undergone invasive experimental procedures is not permitted. Animals may be re-used if their initial experimental use was non-invasive and did not materially affect the animals’ well-being, for example, unused breeding animals or animals that have been used in behavioral or observational studies that do not include the induction of pain or stress. Typically this includes animals used on protocols with a USDA pain category of B or C. Agricultural animals may be re-used in multiple teaching protocols if the protocols involve only procedures that are normally performed in routine husbandry or veterinary care. Cows that have undergone a fistulation procedure should be evaluated as indicated below.
Proposals to re-use animals on protocols with a USDA pain category of D or E should be addressed on a case-by-case basis in consultation with the Attending Veterinarian.
15.30 Fish Counting
Purpose: The University of Vermont is responsible to OLAW (Office of Laboratory Animal Welfare) and USDA for assuring that animal numbers on approved protocols are accounted for. This policy is to provide direction to investigators regarding when they must count zebrafish (Danio species) or other fish species as part of an animal-use protocol.
POLICY: OLAW considers larval and adult forms of fish to be covered by the Public Health Service policy, and both of these forms must be counted. OLAW has made the determination that all stages of zebra fish development greater than 3 days post-fertilization must be described in animal-use protocols. Zebrafish from 4 days post-fertilization onward are considered animals, and therefore must be counted. Other species of fish similarly must be counted after the larvae have emerged from the egg, which will vary by species and incubation conditions.
This policy acknowledges that making exact counts of zebra fish and other fish larval forms is difficult, and allows knowledgeable investigators to rely on experience and expertise to arrive at estimated counts that accurately reflect numbers of zebra fish 4 days post-fertilization as well as other fish species that are used.
Adapted from University of Wisconsin policy, 2012-044-v
15.31 PI Cage Changing Policy for Rodents
Consistent husbandry practices are crucial for the maintenance of animal health and well-being as well as minimizing variables in the research environment. Animal Care staff are trained and experienced in animal husbandry and adhere to strict standards with regards to cage cleanliness, provision of food and water, and documentation of care. When investigators require that laboratory personnel perform routine husbandry tasks in the course of conducting research activities, this requirement must be scientifically justified with appropriate literature search justification in the animal use protocol and approved by the IACUC.
Investigators performing husbandry in their rodent colonies must use the same frequency of cage changes, standards of cleanliness and adequacy of food and water as outlined in Standard Operating Processes used by Animal Care personnel. Investigators must document husbandry activities on the log sheet present in the animal holding room. Animal Care staff will inform research personnel promptly if these standards are not maintained; if an adverse situation (inadequate feed or water, dirty or overcrowded cages) is not corrected within 24 hours, Animal Care staff will provide the necessary remediation.
15.32 Guidelines for Counting Animals Used in Breeding Protocols
OLAW FAQ F-2 (accessed March 15, 2021)
“F. Animal Use and Management
2. Is the IACUC responsible for tracking animal usage?
Although the PHS Policy does not explicitly require a mechanism to track animal usage by investigators, it does require that proposals specify a rationale for the approximate number of animals to be used and be limited to the appropriate number necessary to obtain valid results. This implicitly requires that institutions establish mechanisms to document and monitor numbers of animals acquired and used, including any animals that are euthanatized because they are not needed. Monitoring should not exclude the disposition of animals inadvertently or necessarily produced in excess of the number needed or which do not meet criteria (e.g., genetic) established for the specific study proposal. Institutions have adopted a variety of administrative, electronic, and manual mechanisms to meet institutional needs and PHS Policy requirements.”
PIs must make a thoughtful effort at initial submission of the protocol in estimating the total number of animals that they need considering “wastage” animals in their totals. It is recommended that researchers make a generous estimate of litter sizes in this calculation.
To align with the spirit of the 3Rs when utilizing breeding activities, there must be controls on the numbers of offspring allowed to be produced to meet the objective. Researchers should not be allowing animals to breed unless there are protocols where the pups can be transferred.
Counting Animal Use
Investigators must count and account for all animals used in association with a given protocol.
- Animals purchased from a vendor or imported from a research institution
For example: Twenty animals arrive from a vendor, only 15 were used in the experiment. All 20 animals are counted at their arrival to the Animal Care Facility.
- Animals in a breeding colony
- All breeders and all offspring produced, even if only a sub-set of the offspring were used for actual experimentation will be counted.
For example: Ten mice are produced from a selected mating of one male and one female. However, genotyping reveals that only 2 of the offspring are of the right genotype and those 2 animals are transferred to a research protocol and used for experimentation. All 12 animals (one male, one female, ten offspring) are counted.
- Animals produced from a breeding colony that are transferred to a research protocol are reported and counted on BOTH the breeding colony protocol AND the research protocol. In the example above, 12 animals should be counted on the breeding colony and 2 of these animals will also be counted on the research protocol. Therefore, animals produced on a breeding colony protocol and are not transferred to a research protocol are reported on only the breeding colony protocol.
- Breeding approved within an experimental protocol
- All breeders and all offspring produced, even if only a sub-set of offspring were used for the actual experimentation will be counted.
- Adults and Pre-weaned pups that have been experimentally manipulated
a. All animals used in association with an approved protocol must be counted.
Reference: “Should you count your mice before they’re weaned?” (2006) Lab Animal 35(1), https://grants.nih.gov/grants/olaw/references/laba06v35n1.htm
15.33 Protocol Transition Statement
As of December 2019 the IACUC will be transitioning all previously approved protocols from the InfoEd system to the UVMClick system. It is preferred that the transition coincide with the triennial reviews, resulting in a 3-yr transition period, however that is not acceptable as the InfoEd contract ends prior to that. With an approved InfoEd extension of 6 additional months, the plan is to transition all protocols in one full calendar year. Investigators will have the option of creating and submitting each individual protocol or combining protocols under an umbrella or both individual and umbrella protocols. This will be dependent upon the sponsor and species type.
No changes to currently approved protocols will be allowed as a part of this transition as the planned Designated Review is focused on ensuring that the new protocol record in UVMClick is identical to that which was approved initially in the InfoEd system. Following that principle, the Policy and Procedure Committee does not feel that the transitioning protocols warrant new scientific merit review, statistical review, nor veterinarian review.
All previous protocol documentation of initial and subsequent review prior to transitioning to UVMClick will be maintained in the RPO shared drive. This includes all previous Committee actions including, initial scientific merit, statistical, and veterinarian review. These documents are available to inspectors upon request.
15.34 Sanitation Validation of Equipment in Laboratories
Background
Sanitation of equipment and materials that come into direct contact with laboratory animals is required by federal regulations. Effective sanitation generally requires both cleaning (the mechanical removal of organic debris) and disinfection (the removal or reduction of micro-organisms) of contact surfaces and can be achieved via a variety of methods. Animal caging used on campus is sanitized with automated cagewash equipment and verified to be sanitized before use. Equipment located within laboratory spaces that comes into contact with animals and is sanitized using alternative methods must be verified for effective sanitation. It is important that the methods employed are well defined in your IACUC protocol and periodically evaluated to ensure that sanitation standards are met.
Equipment with direct animal contact includes anesthesia induction chambers, experimental equipment (e.g., elevated plus maze, behavioral chambers), transport containers, urovoid apparatus, metabolic caging, stereotaxic instruments, and surgical platforms.
- Contact surfaces must be sanitized as described in the IACUC protocol.
- Validation of these practices must be performed semi-annually and will be coordinated through the Office of Animal Care Management (OACM). OACM utilizes adenosine triphosphate (ATP) surface test monitoring swabs (i.e., CHARM Sciences, Inc. PocketSwab Plus) and will coordinate with individual laboratories to provide and read swabs.
- Sanitation logs will be maintained by OACM
- For laboratories with multiple pieces of equipment requiring sanitation verification:
- Choose one piece of equipment per semi-annual test cycle to be swabbed per laboratory.
- Equipment should be tested on a rotating basis so that all equipment is eventually swabbed and verified.
- ATP limit threshold for different surfaces/equipment:
- Follow OACM SOP on ATP limit thresholds for different surface types.
- What happens if a swab “fails”?
- If the swab reads higher than the OACM established ATP limit, then the equipment should be re-sanitized and re-tested.
15.35 Animal Acclimation
The Guide for the Care and Use of Laboratory Animals states that newly received animals should be given a period for physiological, psychological, and nutritional acclimation before their use. This allows animals to recover from shipping stress and permits them to adapt to their new surroundings. The length of time for acclimation will depend on the type and duration of animal transportation, the species involved, and the intended use of the animals. This policy will guide the amount of time an animal should be allowed to acclimate to the facility after their arrival from outside sources. Healthy, well-acclimated animals provide reliable and reproducible data.
Animal Acclimation Times
Rodent Species | 3 Days |
Non-Rodent Species | 5-7 Days |
Any Animal Species Used Immediately For Euthanasia And Tissue Harvest | No acclimation period required |
Animals Arriving from Other Institutions | A quarantine period is required and must be coordinated with OACM |
15.36 Policy on Photography or Video Monitoring of Animals
In general, photography or video monitoring of UVM research animals is not allowed, except under the following conditions. Justifiable use of animal photography and video monitoring may include research or housing locations where access presents an increased risk to staff, such as in the ABSL-3 animal facility.
The IACUC has approved the use of video monitoring of animals under the following conditions.
- The principal investigator whose animals are being monitored with a camera is ultimately responsible to ensure these procedures are followed.
- Approval must be received from the IACUC prior to transmitting any video signal.
- A fixed location camera is placed in the animal room and targeted at the desired animal(s).
- A sign on the door of the animal room will indicate that video monitoring is in use.
- Affected personnel will be made aware of the use of video monitoring.
- The camera may be capable of color imaging during normal room lighting and black & white imaging with infrared illumination for “dark hours” imaging for certain circumstances.
- Access to photographic or video images of animals must be limited to a password-protected secure website available to the least number of staff possible, including animal care, veterinary, and research staff.
- The video images may also be accessed 24/7 through a password protected secure website.
- Access to the website will be limited to veterinary staff and appropriate project personnel.
- The website is only available through secure access.
- UVM username and password is required
- Website address is only disclosed to appropriate personnel
- Video System username and password is required
- In addition, a virtual provider network (VPN) which is only available to authorized UVM affiliates, is required for off campus web access.
- Observation of real time video images of animals will enhance our monitoring capabilities, not replace the currently required post procedure animal observations.
- Photography or video monitoring of animals must be justified and pre-approved by the IACUC and the Attending Veterinarian.
- An audio system may be added to greater enhance monitoring capabilities. Associated personnel must be notified prior to activating an audio system.
- In approved instances where remote monitoring is used for husbandry checks, animals should be observed in-person at least weekly.
15.37 Use of Radios and Other Sound Generating Devices in Animal Rooms
Purpose:
The purpose of this policy is to define the acceptable use of radios and other sound generating devices within animal housing areas.
Background:
Exposure to loud background sounds can have both auditory and non-auditory effects on animals. Many species can hear frequencies of sound that are inaudible to humans, thus the potential effects of sound-producing instruments such as radios must be carefully considered. When at all possible, activities that might be considered noisy should be conducted in rooms or areas separate from those used for animal housing.
Per the Guide, page 50: “Radios, alarms, and other sound generators should not be used in animal rooms unless they are part of an approved protocol or enrichment program.
Any radios or sound generators used should be switched off at the end of the working day to minimize associated adverse physiologic changes (Baldwin 2007).”
Policy:
Playing radios or other sound generating devices in animal rooms is not permitted unless it is part of the facility SOP.
15.38 Research in Foreign Countries
Research conducted by the Institution’s investigators in foreign countries falls under the Institution’s purview and guidelines. Regardless of the setting, the standards for ethical and responsible use of animals in research will not be relaxed even if different customs prevail. All animal-based research conducted in foreign countries is subject to IACUC review. This includes the use of animals in foreign research institutions, and fieldwork involving either domestic or wild animals. Research projects must be approved by the local equivalent of an IACUC before they are initiated. Where there is no equivalent board or group, investigators must rely on local experts or community leaders to provide approval. The IACUC requires documentation of this local approval, as well as documentation of any necessary permits, before granting final approval for the project. With regard to activities supported by PHS funds, foreign institutions that serve as performance sites must also have Assurances on file with OLAW.
Research involving foreign collaborators is subject to all applicable federal/state laws and institutional policies.
