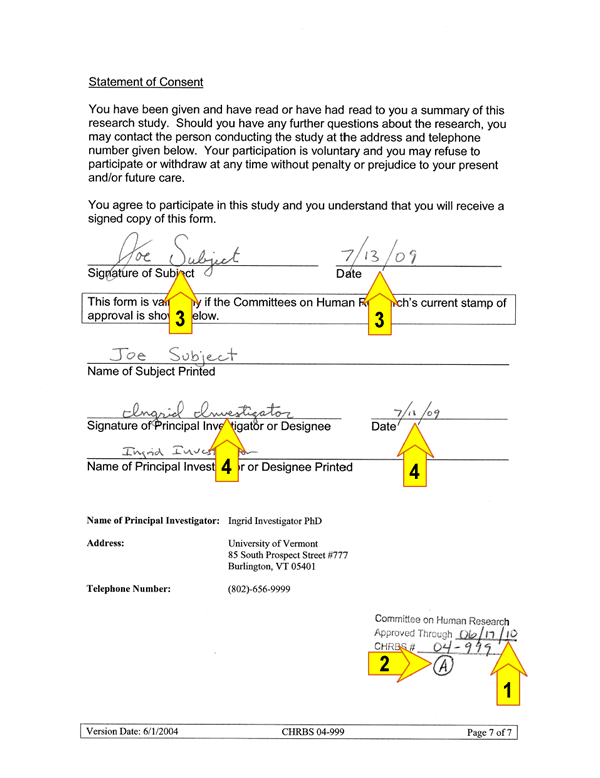
Obtaining Legally Effective Informed Consent
Before research activities may begin there are FIVE key elements that must be completed and/or verified (referred to as a fully executed consent):
1 ) Expiration date on the consent form is current
2 ) Consent form, if applicable, includes all previous amendments
3 ) Signed and dated by the subject where indicated
4 ) Signed and dated by the investigator or designee where indicated5) All blanks are appropriately completed
An example of a fully executed consent document (key elements are numbered):
