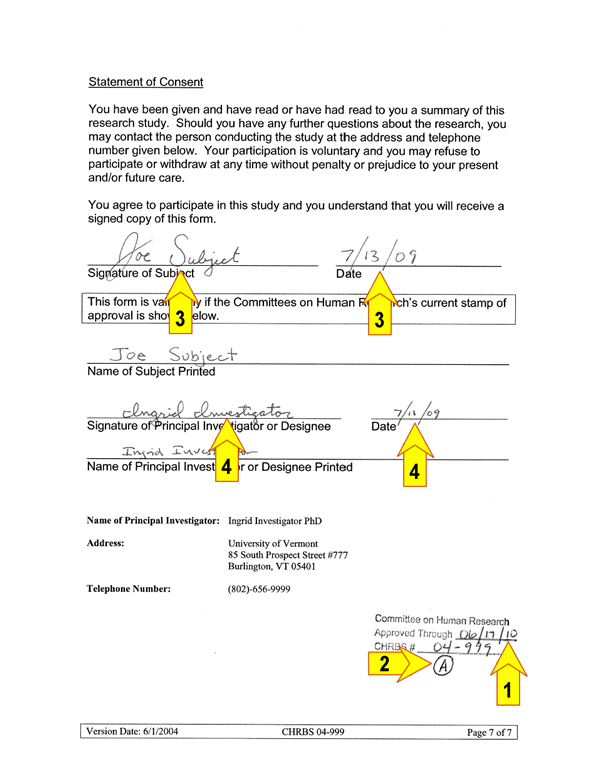
Obtaining Legally Effective Informed Consent
Before research activities may begin there are FIVE key elements that must be completed and/or verified (referred to as a fully executed consent):
1 ) Expiration date is current
2 ) Consent form is the most current version
3 ) Signed and dated by the subject on the appropriate line
4 ) Signed and dated by the member of the team indicated on the form, i.e. PI (or designee) on the appropriate line and at the time consent was obtained5) All blanks are appropriately filled in and there are no handwritten changes made to the document
An example of a fully executed consent document (key elements are numbered):
