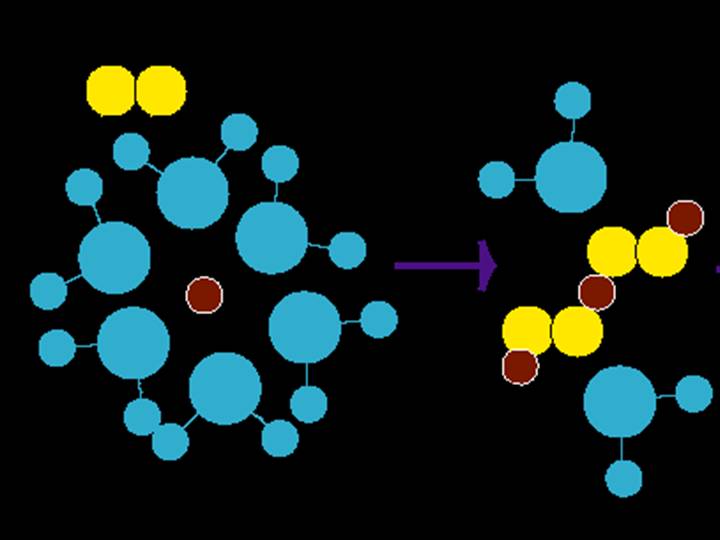
The aggregation of molecules from free ions in solution (or simple aqueous complexes) towards the formation of nanocrystals r bulk minerals must pass through an intermediate phase where a number of molecules are together, but the aggregate size of the molecules is not quite large enough to be considered a solid phase. the size of this can vary between a few molecules to hundreds, depending on how we decide on the size of the smallest crystalline (nanocrystalline really) phase.

Figure - Representation of intial cluster formation from Fe2+(H2O)6 and S22-
Molecular clusters of metals and sulfides have bee identified for Cu, Pb, Zn, Fe, and Mn in solutions. We are working most closely with FeS(aq) clusters and the role of Mn as a part of those clusters. Voltammetric analyses is the only known method for in situ detection of these species in solution - commen colorimetric and spectroscopic methods employed to analyze solutions would not identify these clusters, but rather include the metal and sulfide as free metal ions or hydrogen sulifde species, respectively.
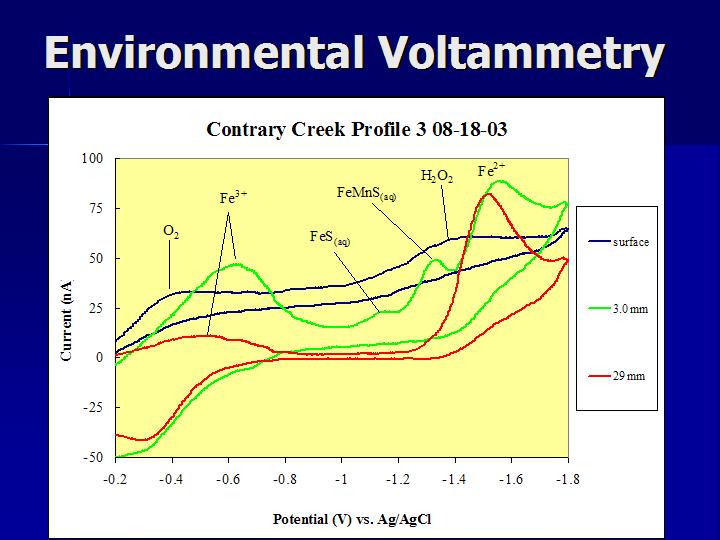
Figure - Voltammograms collected and overlain from a freshwater marsh area near Contrary Creek in Virginia. Peaks labeled for FeS(aq) and the FeMnS(aq) cluster are shown.