Step 1: How to Access CITI
I would like to use my UVM Net ID
All UVM students, faculty and staff are issued a UVM Net ID, but there are many UVMMC (non-UVM) research key personnel who do not have a UVM Net ID. A UVM Net ID can be issued to UVMMC personnel to access the CITI Program Training. Processing time can take 1-2 business days. Learn more about obtaining a UVM Net ID.
- Once you have an active UVM Net ID, then you may open the browser and go to the CITI Login Page (opens in a new window).
- Click “Log In” which is located at the top right of the CITI page.
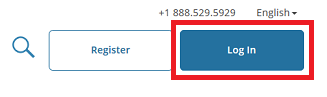
- Then, click "Log In Through My Institution" to view the organizations listed to use "Single Sign On" (SSO) for CITI Program access, scroll down to click on “University of Vermont”...
- and use your UVM Net ID and password to sign in.
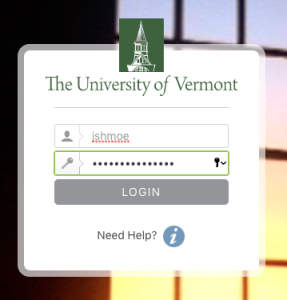
- If you receive an error message at sign in, then please refer to instructions on
How to make sure you have a UVM Net ID
OR
How to create a separate account through CITI (Register).
- and use your UVM Net ID and password to sign in.
- If you haven’t associated your UVM account with CITI, you will be prompted to choose whether you already have a CITI Program account or if you don't have a CITI Program account.
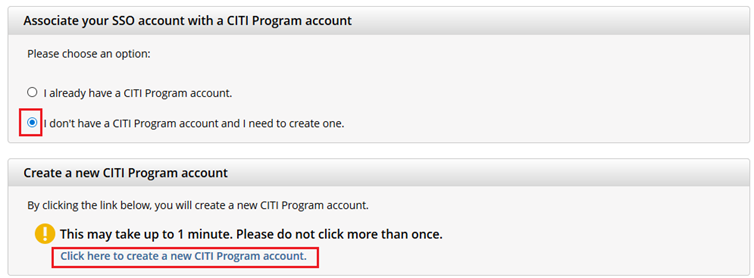
- If you have set up a CITI account in the past, choose the first option
"I already have a CITI Program account" and follow the instructions to link your UVM account. - Otherwise, choose the second option and select
“I don’t have a CITI Program account and I need to create one.” - If you do not receive these prompts, then proceed to step 5.
- If you have set up a CITI account in the past, choose the first option
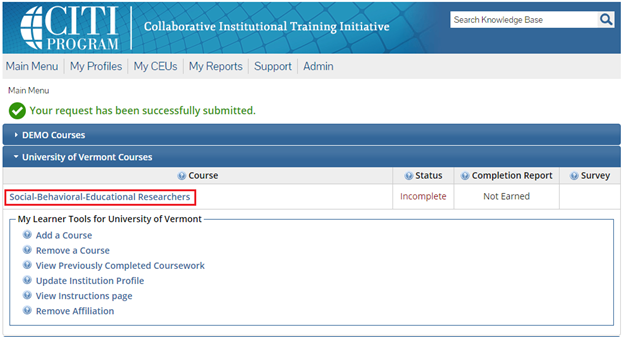
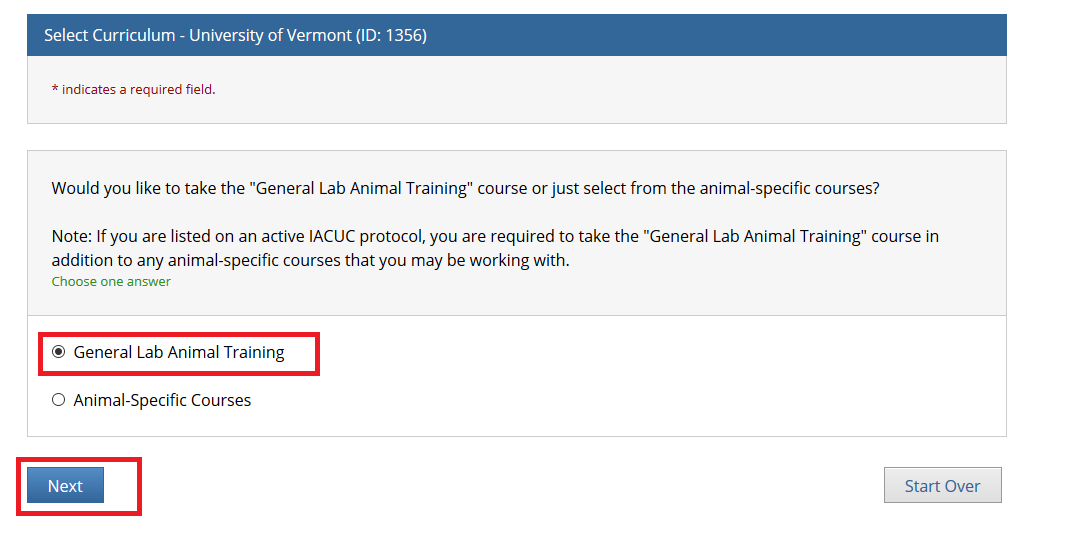
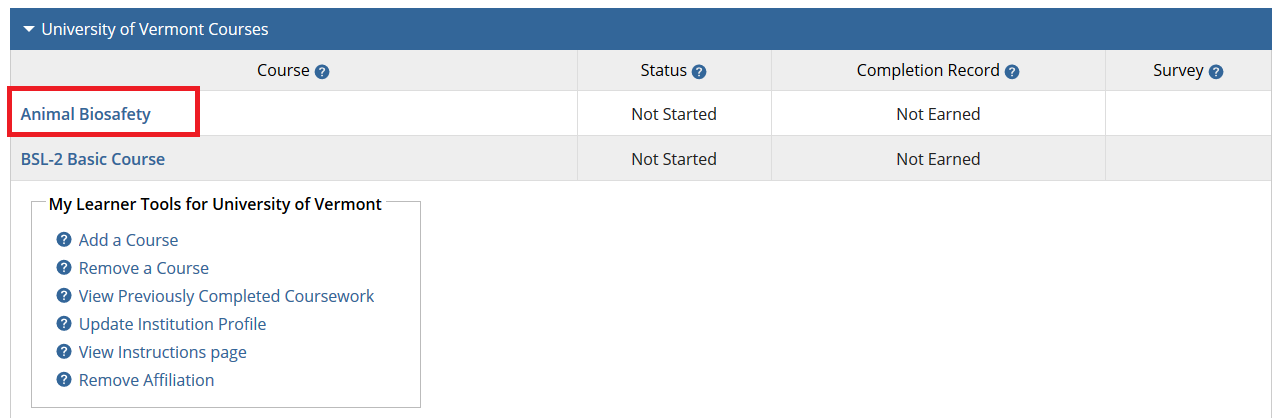
- From the Main Menu, select "View Courses."
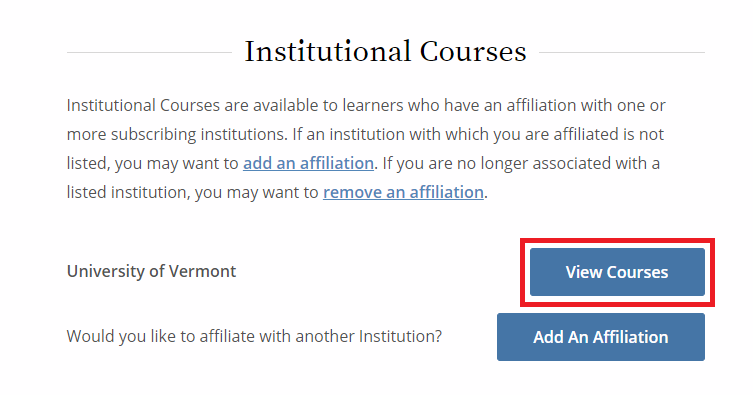
- Then, select “Add a Course.”
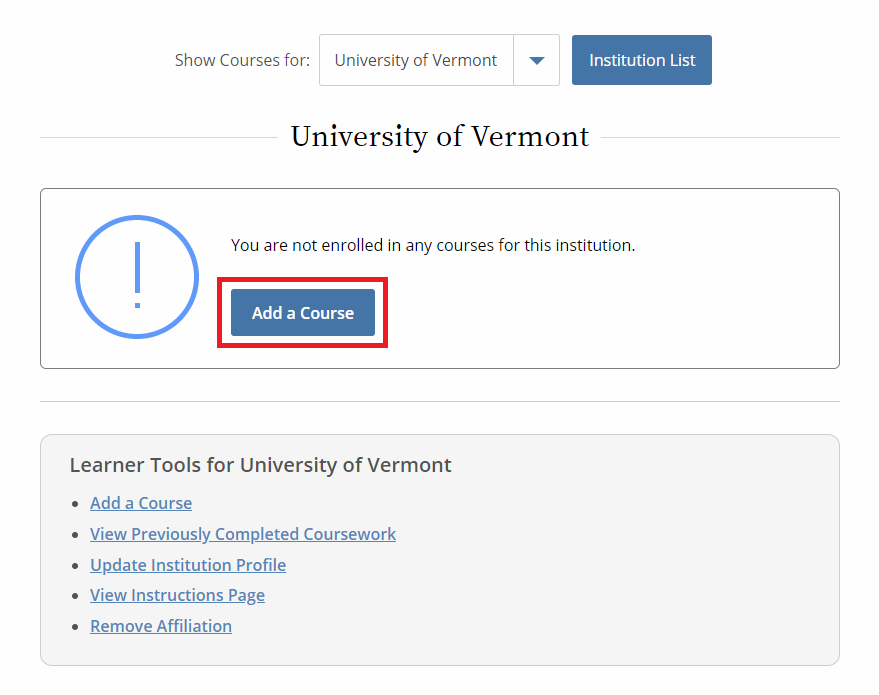
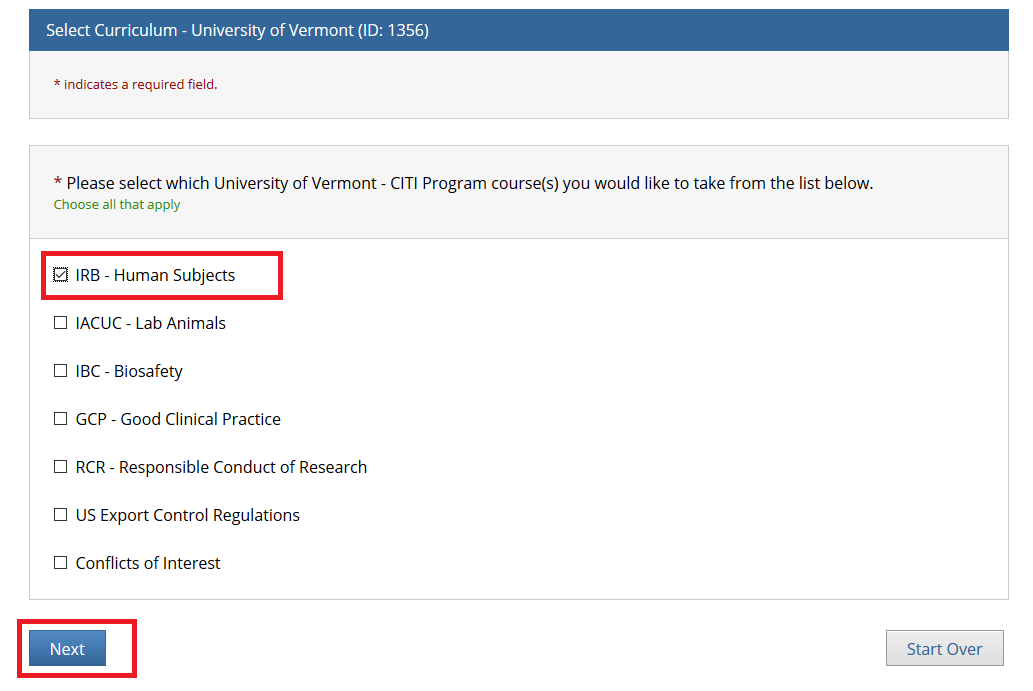
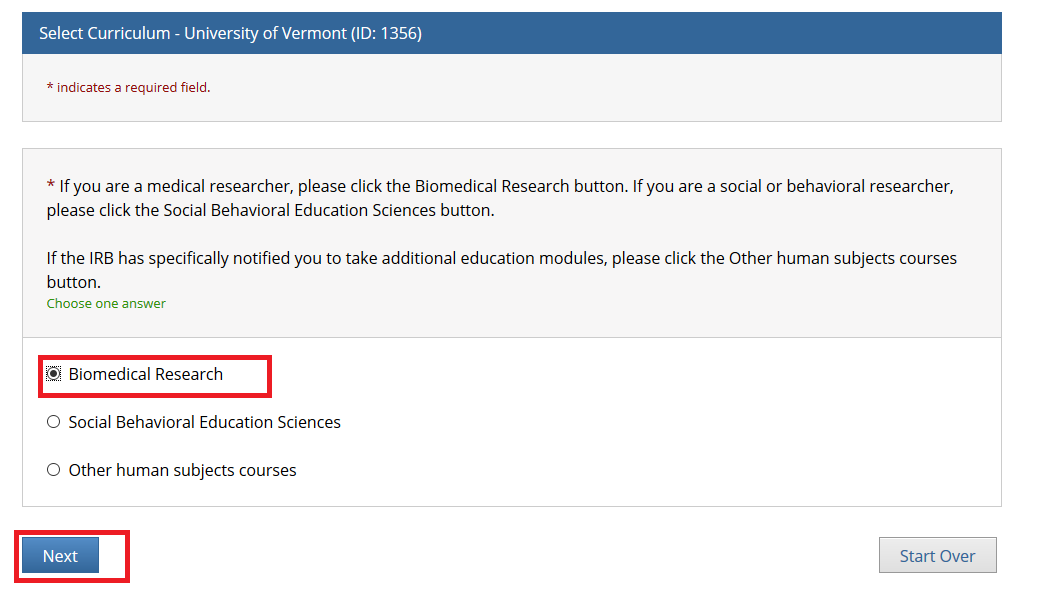
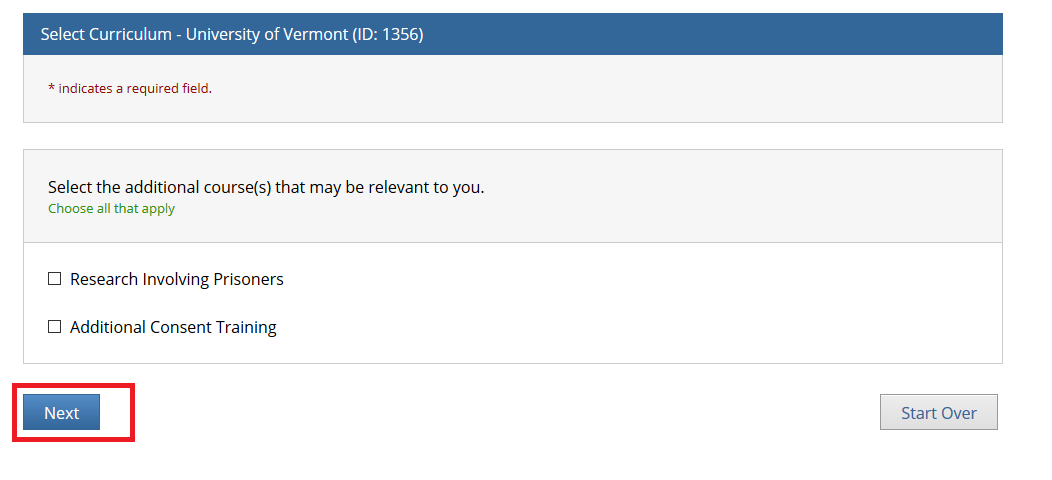
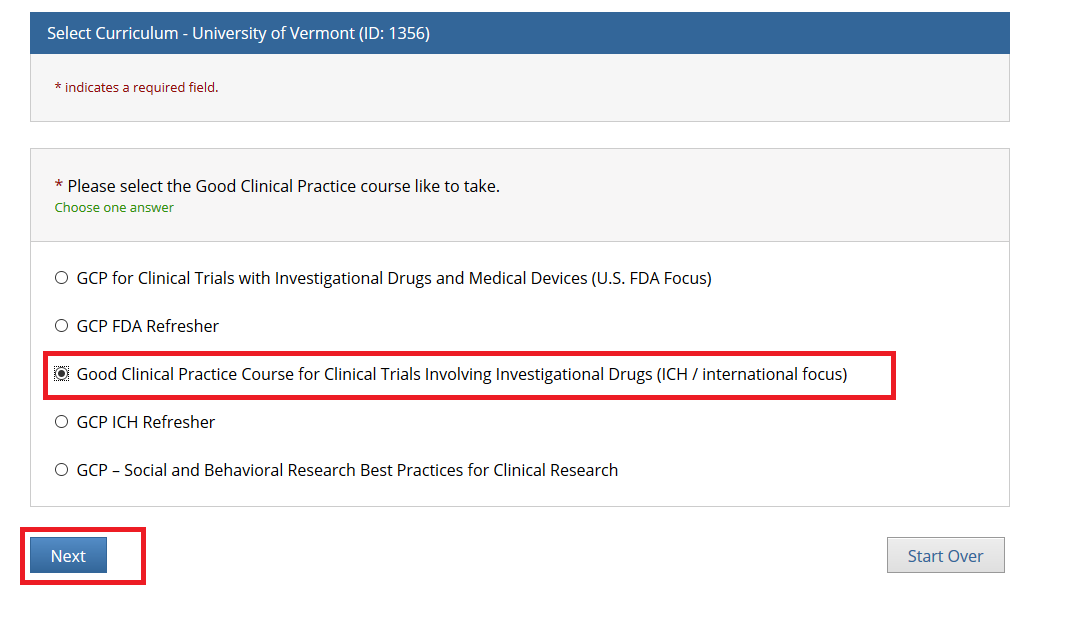
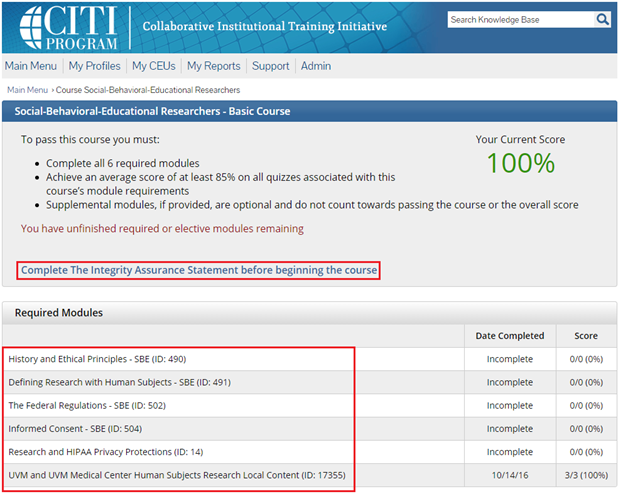
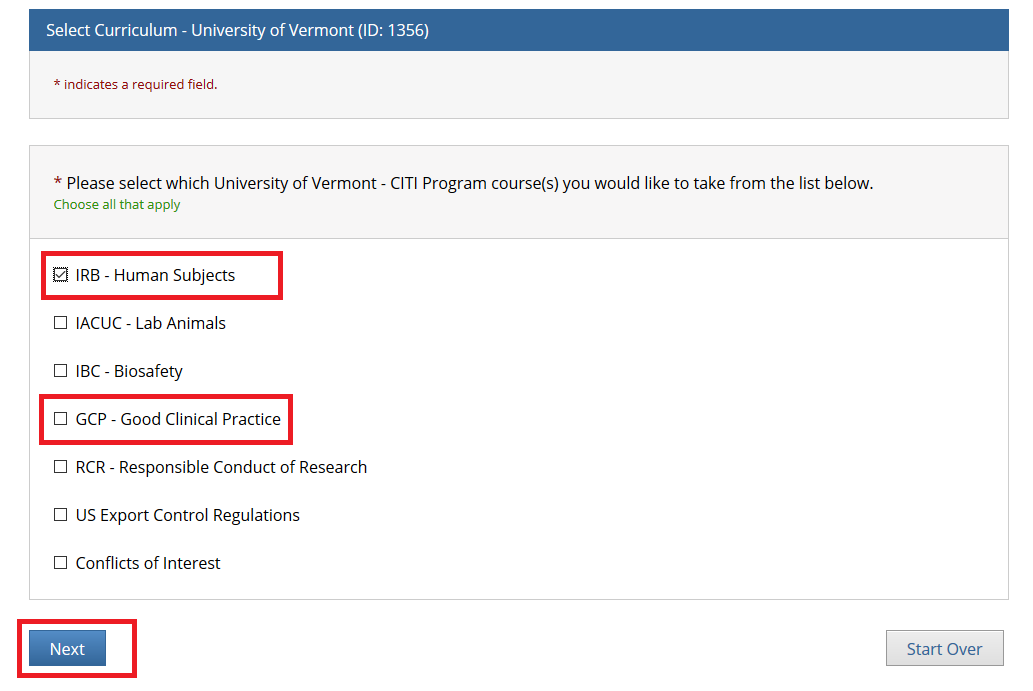
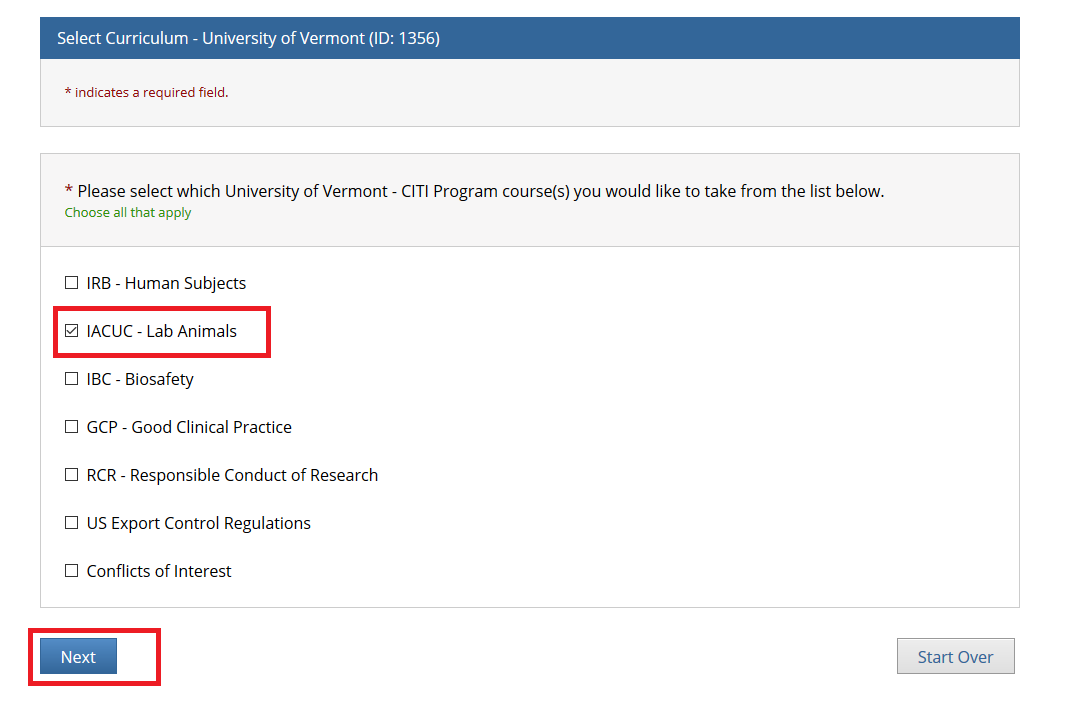
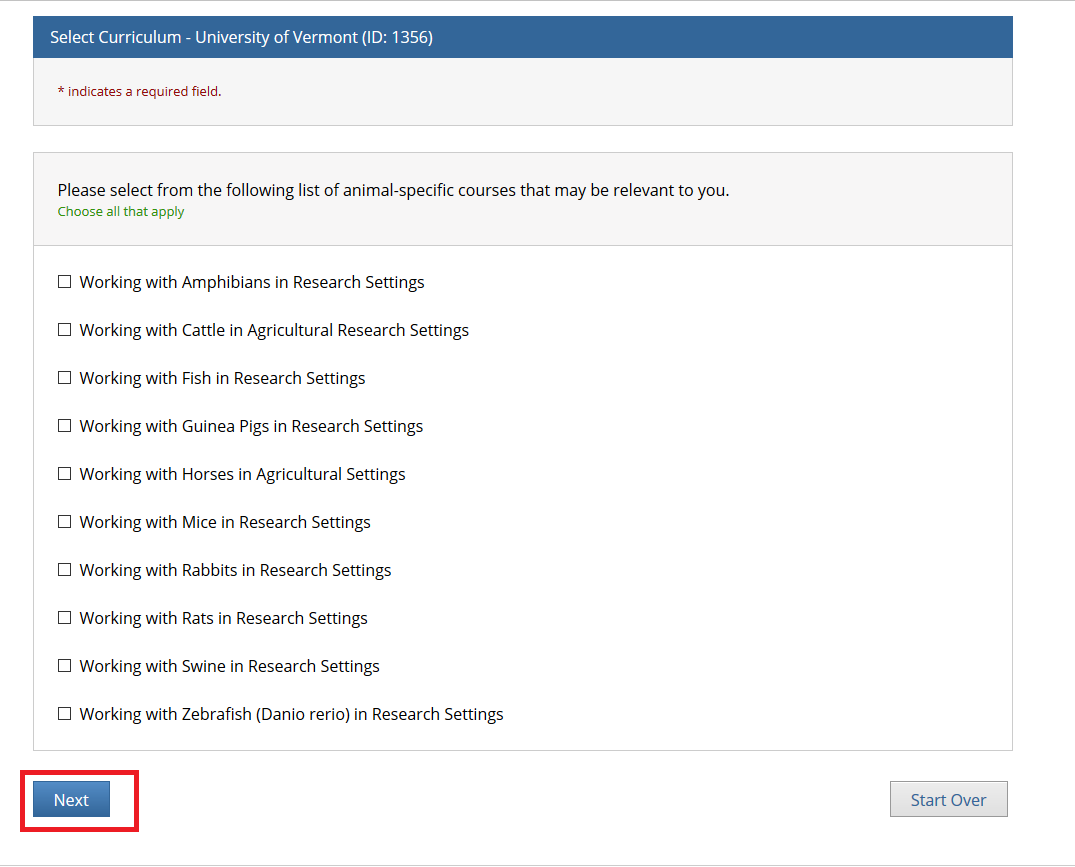
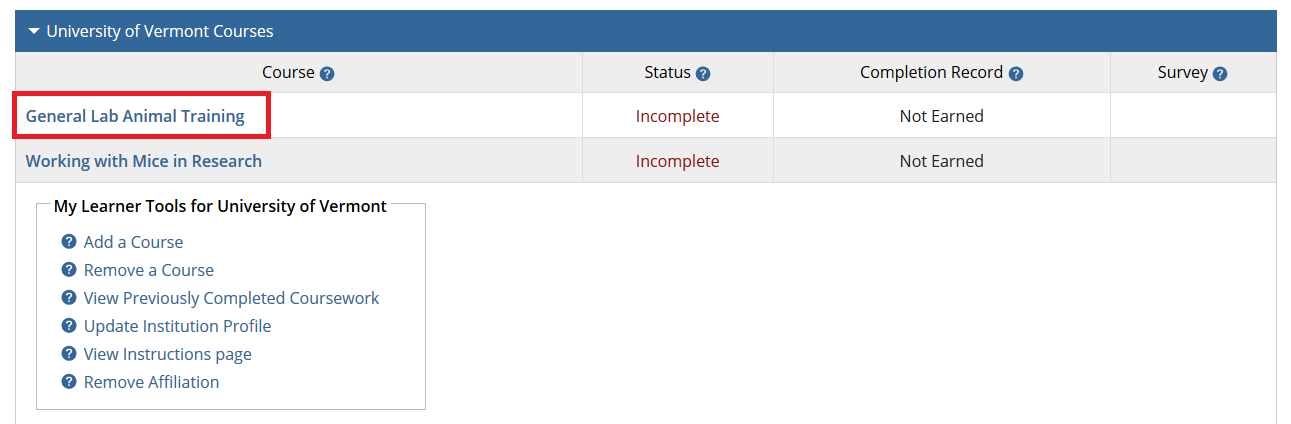
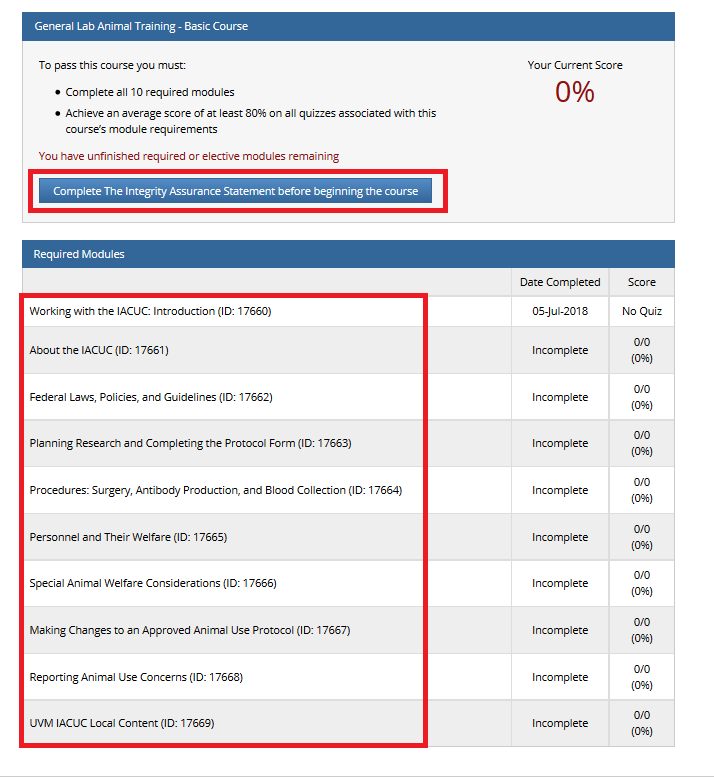
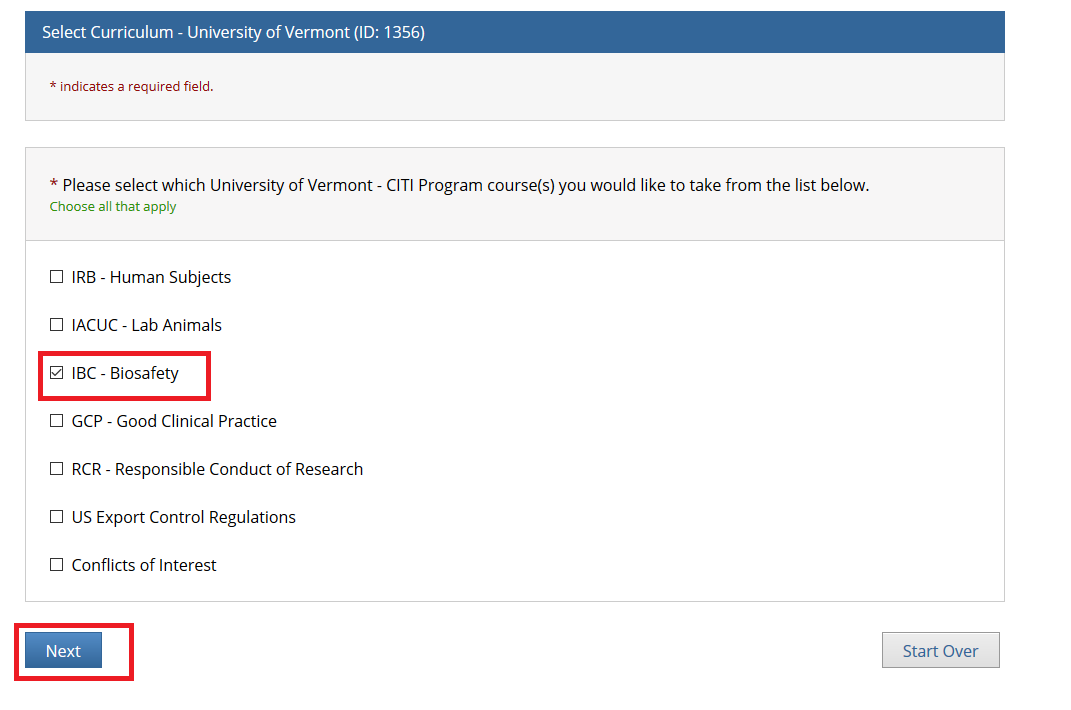
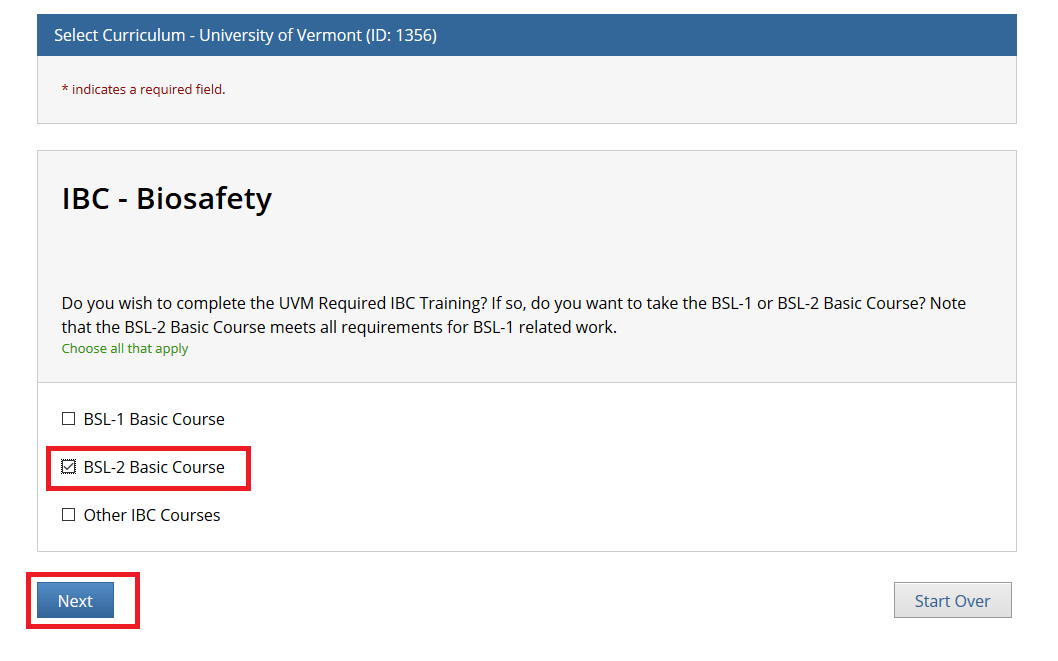
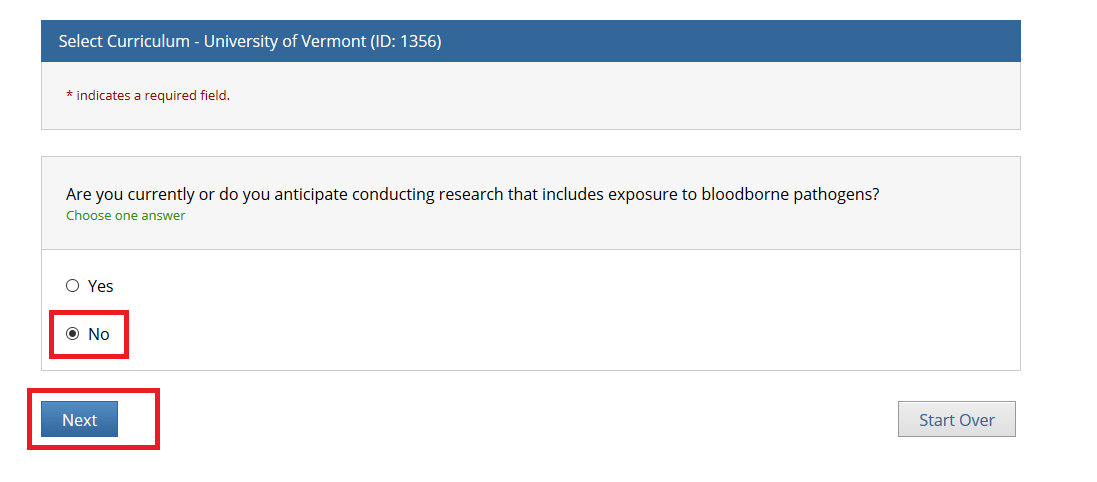
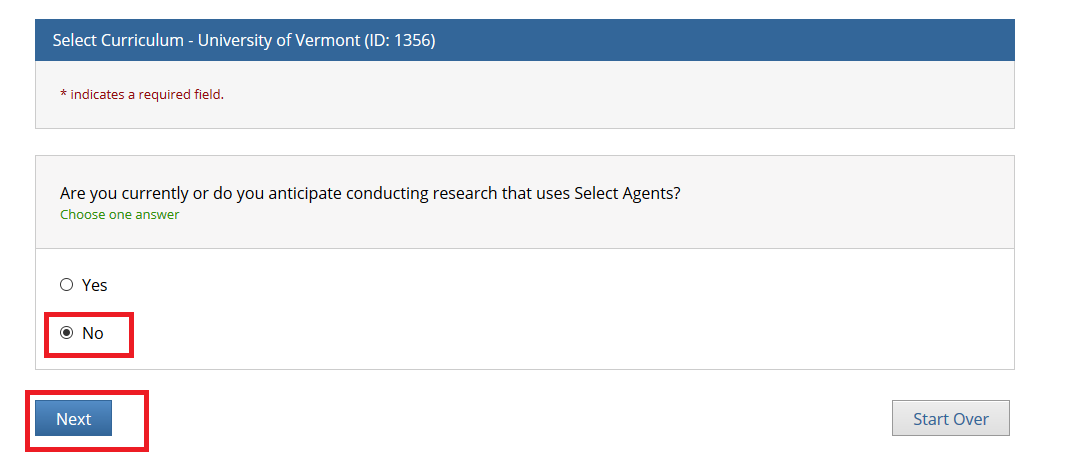
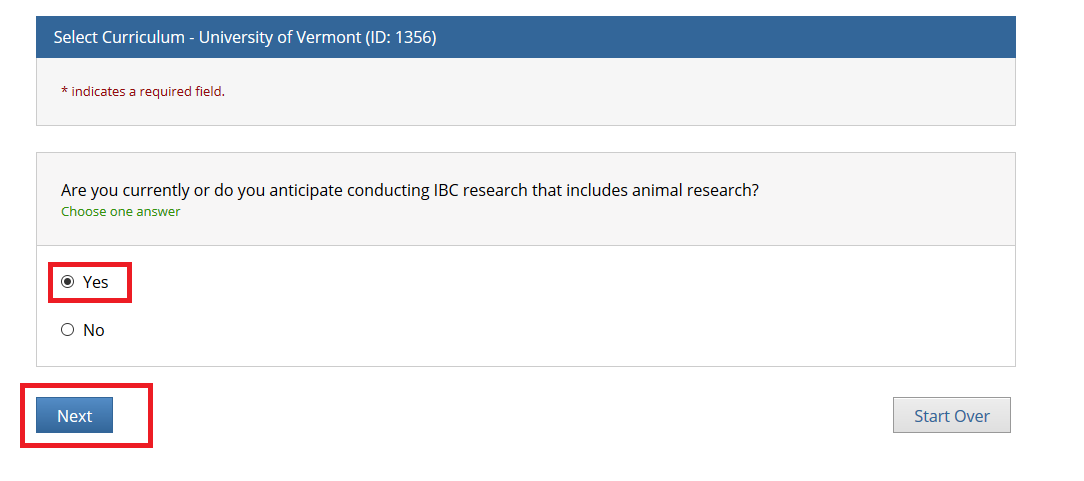
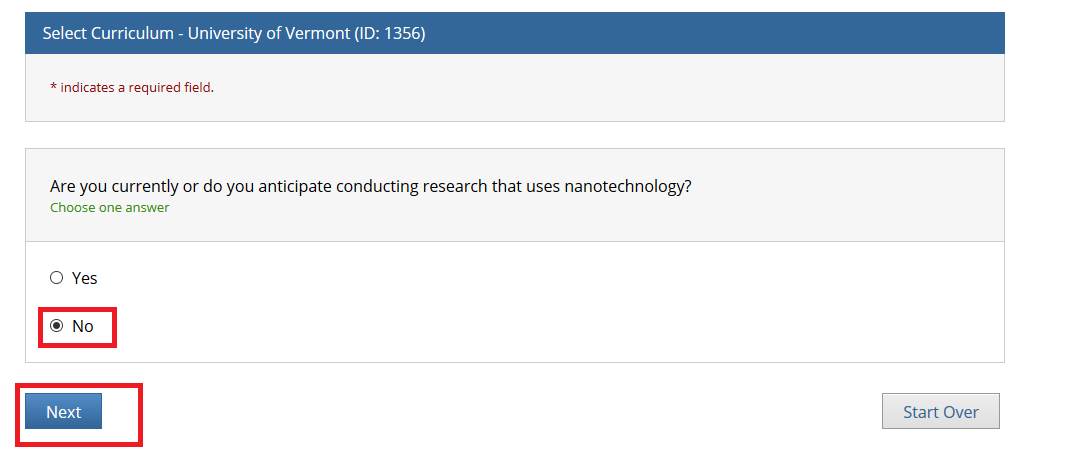
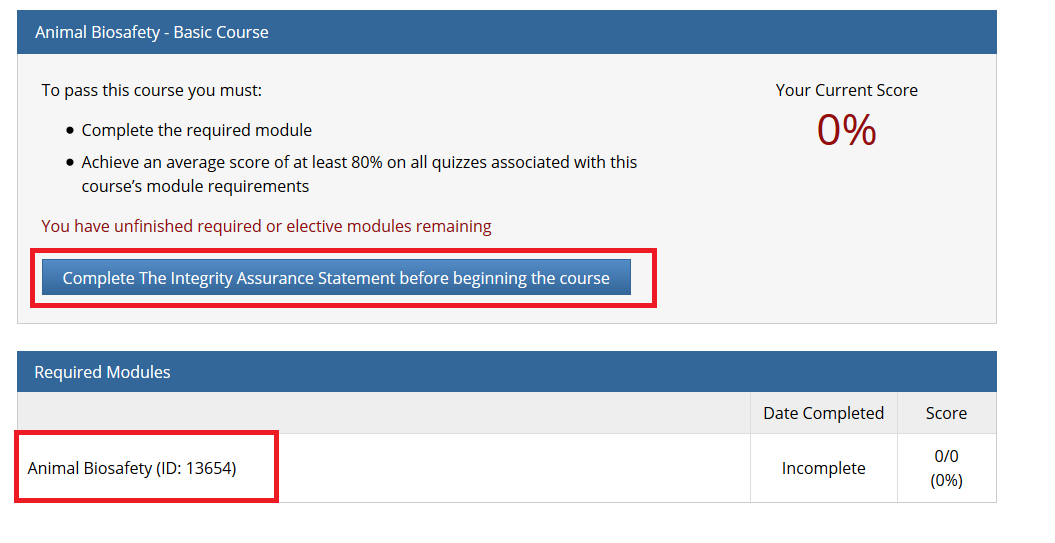
- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC.
A Guide to Getting Started video is also available.
- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC.
- Then, select “Add a Course.”
I would like to Register
This allows UVM affiliates to access the CITI training on the same day without a UVM Net ID.
- Go to the main CITI Login Page (opens in a new window) and click the "Register" button at the top right of the page.
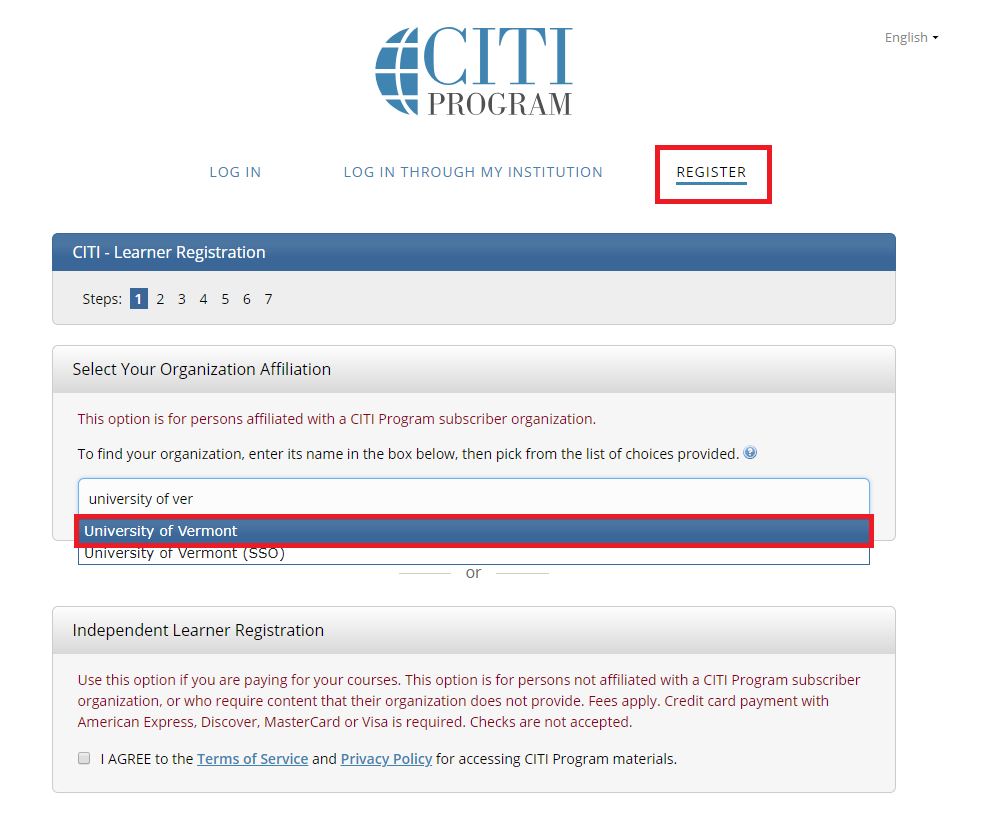
- Step 1: Select Your Organization Affiliation - "University of Vermont".
- Agree to Terms of Service
- Affirm that you are a UVM affiliate
- Select "Continue To Create Your CITI Program Username/Password"
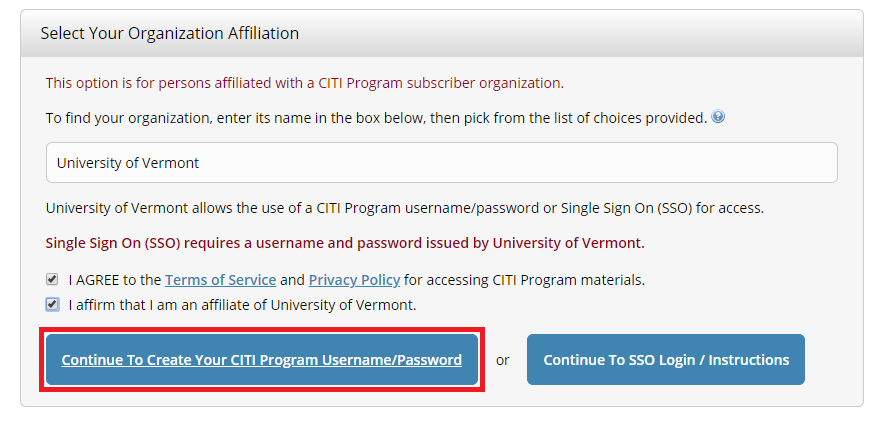
- Complete the Remaining Registration Steps 2 -7.
- Note: Step 5 will ask about receiving Continuing Education Unit credits. Please check "No" to this option and complete the remaining registration steps.
- Once you Register through CITI, then you may open the browser and go to the CITI Login Page (opens in a new window).

- Click “Log In” which is located at the top right of the CITI page.

- Then, enter the Username and Password for the CITI Program account you just created and click the blue "Log In" button.

- From the Main Menu, select "View Courses."

- Then, select “Add a Course.”

- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC.
A Guide to Getting Started video is also available.
- Note: Following this step, please proceed to the course specific instructions below for IRB, IACUC and IBC.
- Then, select “Add a Course.”