Hondal Research Group
Overview: My research group is interested in the redox chemistry/biology of sulfur and selenium. For many years we have focused on the chemistry/biology of selenocysteine, the 21st amino acid in the genetic code and a key question that we have focused on is “Why selenocysteine?”. Some possible answers that we and others have provided is that selenocysteine confers the ability: (1) to resist irreversible oxidation and inactivation to enzymes, (2) to resist permanent electrophilic modification, and (3) catalyze one-electron reductions. A more recent focus has been the chemistry/biology of ergothioneine, a potential vitamin for humans. Ergothioneine is an amino acid derivative synthesized by fungi and some bacteria. It’s true biochemical function in humans has not yet been elucidated, but low levels of ergothioneine are associated with a higher risk of cardiovascular disease, dementia, and frailty in the elderly. We recently discovered that selenocysteine-containing thioredoxin reductase reduces oxidized forms of ergothioneine. Thus, there is a potential link between selenium and ergothioneine.
1. Redox biology of ergothioneine/2-thiohistidine in peptides.
As protein biochemists, we are interested in ways of utilizing the unique chemical properties of ergothioneine. To this end, we recently published a method for incorporating an analogue of ergothioneine, 2-thiohistidine, into peptides. We call this idea “ergothioneine in a peptide. Please see: Jenny KA, Ruggles EL, Liptak MD, Masterson DS & Hondal RJ. (2021) Ergothioneine in a peptide: Substitution of histidine with 2-thiohistidine in bioactive peptides. J. Pept. Sci. e3339.
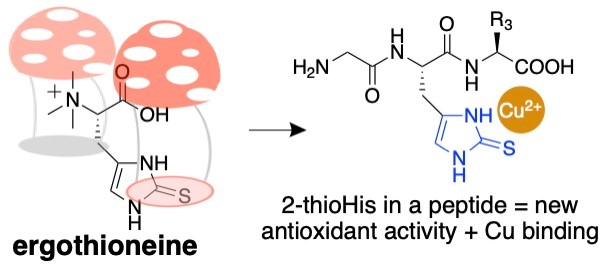
Figure 1: Ergothioneine in a peptide.
Since 2-thiohistidine is a fusion of the amino acids cysteine and histidine, we envision that 2-thiohistidine can replace histidine residues in peptides and proteins when the histidine residue has an antioxidant function. One such example is GHK-tripeptide. This peptide is released from collagen after proteolysis in response to tissue injury. It is widely used in the cosmetic industry. We have synthesized an analogue of GHK-tripeptide in which we replaced the histidine residue with 2-thiohistidine. The analogue has a remarkable gain of antioxidant function.
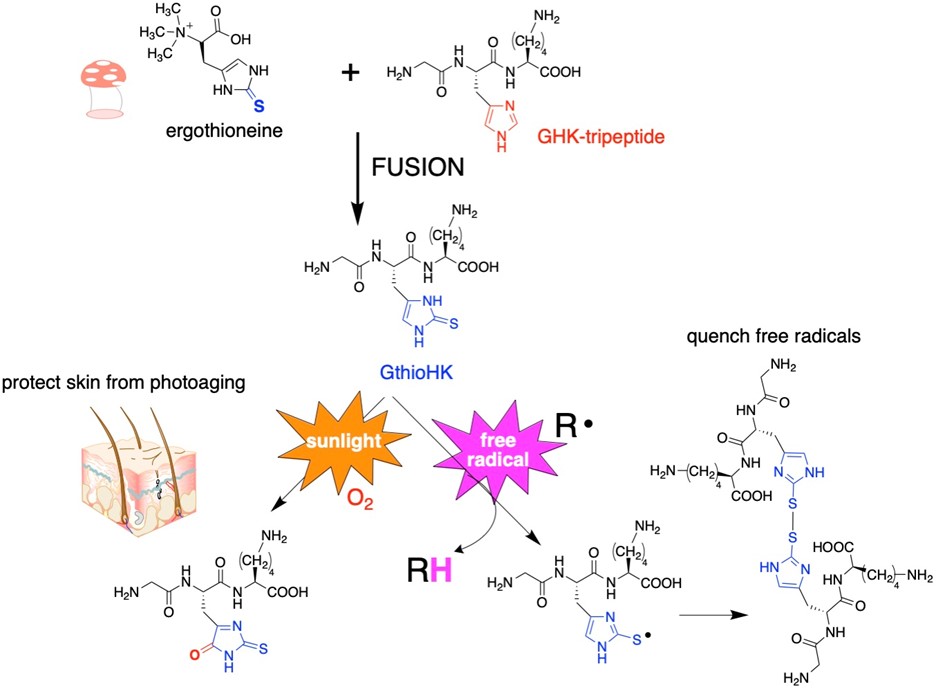
Figure 2: Replacement of histidine in GHK-tripeptide with 2-thiohistidine.
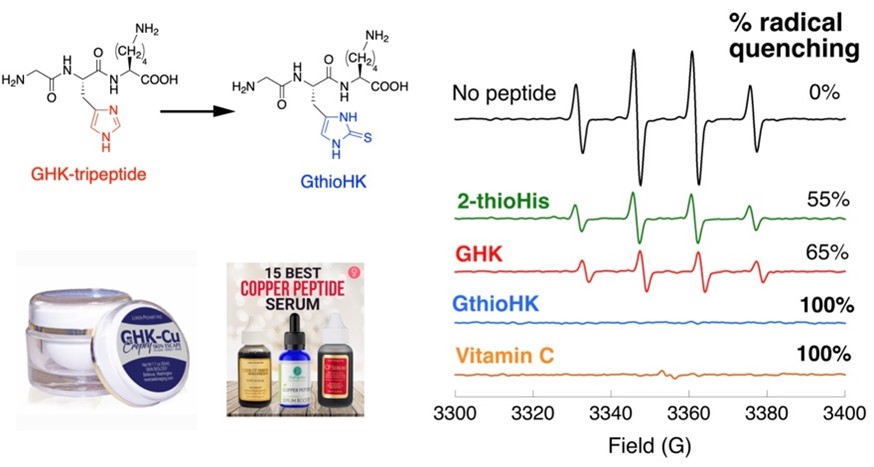
Figure 3: We replaced the His residue of GHK with 2TH. To measure the ability of the analogue to quench hydroxyl radical, we chose to do an EPR experiment, which measures the signal of a free radical. When the free radical is quenched, the signal decreases. Total loss of signal results for both GthioHK and Vit C as shown, indicating our peptide is a comparable antioxidant. It is much better than GHK and the free amino acid.
We are currently working on biotechnological applications of the use of 2-thiohistidine in peptides, similar to the GHK-tripeptide example. Contact me if you are interested in learning more about this project.
2. Chemical/biological relationship between selenium and ergothioneine.
Recently, we discovered that oxidized forms of ergothioneine can be reduced by selenocysteine-containing thioredoxin reductase. Thioredoxin reductase is part of the “thioredoxin system”, one of the major cellular antioxidant systems of the cell. We have yet to fully explore the biological significance of our in vitro discovery. This is an ongoing area of research in the lab. Please see: Jenny KA, Mose G, Haupt DJ, & Hondal RJ. (2022) Oxidized forms of ergothioneine are substrates for mammalian thioredoxin reductase. Antioxidants 11, 185.
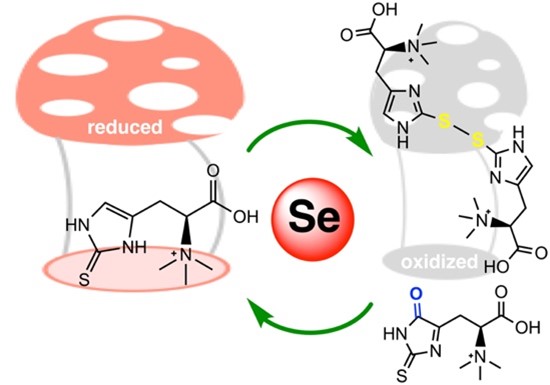
Figure 4: Ergothioneine-disulfide and 5-oxo-ergothioneine are oxidized forms of ergothioneine that can be recycled back to the reduced form by thioredoxin reductase.
3. Antioxidant peptides that target mitochondria.
A typical phospholipid such as phosphatidylcholine (PC) consists of a glycerol backbone esterified with two fatty acids to the C1 and C2 hydroxyl groups. The C3 hydroxyl group attaches the headgroup (choline in the case of PC). Cardiolipin is much different as it contains a central glycerol backbone attached to two phospholipids. The two phospholipids are 1,2-dilinoleoyl-sn-glycero-3-phosphate. Thus, cardiolipin contains four linoleic acids, which is essential to its function as this imparts its characteristic cone shape that is important in forming the cristae of the mitochondria. Linoleic acid is a dietary essential w-6 fatty acid. Peroxidation of cardiolipin leads to mitochondrial dysfunction and loss of ATP production. Mitochondrial dysfunction, associated with increased reactive oxygen species (ROS) production, is an underlying common driver of numerous diseases including: myocardial ischemia/reperfusion injury, diabetes, Parkinson’s Disease, Alzheimer’s Disease, and preeclampsia.
We have been working on peptides that target cardiolipin and protects the mitochondria from lipid peroxidation, thereby preventing mitochondrial dysfunction as shown in Figure 5.
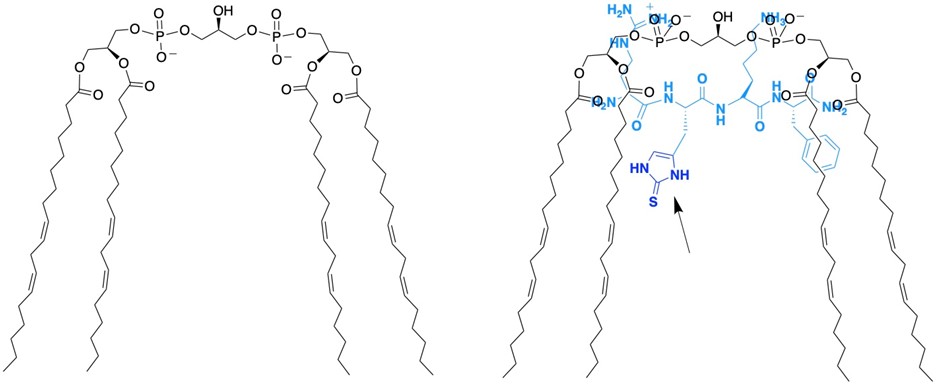
Figure 5: (left) Cardiolipin, an important lipid found in mitochondria that is responsible for the curvature of the inner membrane. (right) We have developed a peptide that targets cardiolipin that contains 2-thiohistidine and protects it from oxidation. The 2-thiohistidine residue is shown by the arrow.
4. Selenium in biological molecules.
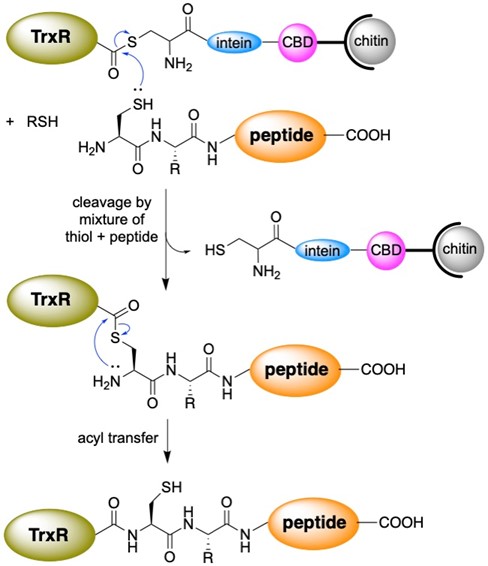
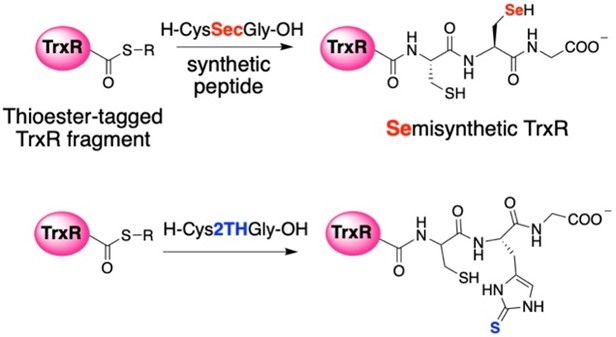
While we have recently begun to study 2-selenouridine, a nucleobase that is found in tRNA in certain bacterial species, most of our work has focused on selenocysteine-containing enzymes, especially thioredoxin reductase. Selenocysteine is encoded in the DNA by a stop codon, TGA. This makes heterologous expression of selenocysteine in bacteria difficult. To overcome this barrier, we use a sophisticated protein engineering technique called “intein-mediated peptide ligation”. Thioredoxin reductase is an enzyme consisting of 490 amino acids, with selenocysteine occupying the penultimate position. To produce the enzyme, we express amino acids 1-487 in E.coli as a fusion protein next to an intein. After isolating the protein by affinity purification, we cleave the truncated enzyme from the intein by thiolysis as shown in Figure 6. This creates a protein with a C-terminal thioester. The thioester can be attacked by a peptide containing an N-terminal cysteine. In the lab, we synthesize a selenocysteine-containing peptide, ligate it to the enzyme, and create a semisynthetic enzyme. This allows us to insert a number of non-natural amino acids into proteins, especially thioredoxin reductase. Recently, we created a mutant of thioredoxin reductase that contains 2-thiohistidine as shown in Figure 7! We have used this technique to study the mechanism of thioredoxin reductase at a high level.
|
Figure 7: Creation of semisynthetic thioredoxin reductase (TrxR) enzymes. Using semisynthesis, we can insert selenosyteine and other non-natural amino acids such as 2-thiohistidine as shown here. | |
Figure 6: Use of intein-mediated peptide ligation to create semisynthetic proteins such as thioredoxin reductase (TrxR).
|
| |