

BIOLOGICAL CONTROL OF ANOPLOHORA GLABRIPENNIS
MOTSCH.:
A SYNTHESIS OF CURRENT RESEARCH PROGRAMS
Investigations of Natural Enemies for Biocontrol of Anoplophora glabripennis (Motsch.)
BIOLOGICAL CONTROL OF ANOPLOHORA GLABRIPENNIS MOTSCH.:
A SYNTHESIS OF CURRENT RESEARCH PROGRAMS
Michael T. Smith1, Yang, Zhong-qi2, Franck Hérard3, Roger Fuester1,
Leah Bauer4, Leellen Solter5, Melody Keena6 and Vince D'Amico6
1Beneficial Insects Introductory Research Laboratory, USDA, ARS, Newark, DE2Research Institute of Forest Ecology, Environment and Protection
Chinese Academy of Forestry, Beijing, CHINA3 European Biological Control Laboratory, USDA, ARS, Montpellier, France
4 USDA, USFS, E. Lansing, MI
5 Illinois Natural History Survey, Champaign, IL
6 USDA, USFS, Hamden, CT
IntroductionAnoplophora glabripennis Motschulsky (Asian longhorned beetle) (ALB) (Coleoptera: Cerambycidae: Lamiinae: Lamiini), is among a group of high-risk exotic woodborers native to Asia, specifically China and Korea (Nowak et al. 2001). In China, A. glabripennis is considered one of the most important forest pests, having been reported from 25 provinces and extending from 21o- 43o N Latitude and 100o?127o E Longitude (Yan 1985). This region extends across climatic zones that correspond to the climatic zones in North America from southern Mexico to the Great Lakes, and includes virtually all of eastern U.S. Feeding by larvae in the cambium and xylem causes widespread mortality among many deciduous broadleaf tree species in China (Yang et al., 1995), particularly Populus spp., Salix spp. and Ulmus spp. (Xiao 1992).
Within the U.S., A. glabripennis has been intercepted in 14 states, but established infestations are currently only known to exist in New York City and on Long Island (first discovered in 1996), and in Chicago, Illinois (first discovered in 1998). Utilizing the most effective method currently proven to limit its spread, approximately 5,286 and 1,509 infested trees have been located, cut and removed in the New York and Chicago infestations, respectively, as of May 2001 (US Forest Service 2001). Furthermore, A. glabripennis has thus far been reported to attack 18 deciduous tree species in 12 genera within these two U.S. infestations (Cavey et al. 1998; USFS 2001). Most notably among these are maples (Acer spp.). In addition to the ability to attack and kill apparently healthy trees, A. glabripennis also structurally weakens trees, which poses a danger to pedestrians and vehicles from falling limbs or trees.
Although quarantines and eradication programs exist in New York and Chicago, A. glabripennis possesses the potential for introduction into the urban, suburban and forest landscapes, particularly in eastern U.S. Based upon field data from nine U.S. cities, national tree cover data and proposed host preference of A. glabripennis, the estimated potential tree resources at risk to A. glabripennis attack ranges from 12 to 61% of the city tree population, with an estimated value of $72 million-$23 billion per city. The corresponding loss in canopy cover that would occur if all preferred hosts were killed ranges from 13-68%, with an estimated maximum potential impact of 34.9% of total canopy cover, 30.3% tree mortality (1.2 billion trees), and value loss of $669 billion (Nowak et al. 2001).
Therefore, efforts to develop control strategies that represent alternatives to the felling and chipping of infested trees were initiated within the past three years. Included among these are biological control strategies, which are the focus of this paper. These strategies have two broad objectives. The first objective is focused on the development of mass rearing and mass production technologies, coupled with inundative release and application technologies, respectively, for various biological control agents found to be effective against A. glabripennis. The resulting technologies are intended to complement existing or other currently developing technologies (i.e. insecticidal controls) for use in the eradication program. The second objective is to develop technologies that could be utilized in managing A. glabripennis populations should eradication fail to succeed. As such, this paper will provide an update on the biological control research, with the exception of fungal pathogens, which is the subject of a companion paper in these proceedings.
Research Summaries
Nematodes (Solter and Keena). Entomopathogenic nematodes may offer an alternative and/or complementary method for the control of ALB, specifically targeting the larval and or pupal stages. Therefore, four entomopathogenic rhabditoid nematode species, Steinernema carpocapsae, Heterorhabditis bacteriophora, H. indica, and H. marelatus, were tested for their ability to kill and reproduce in larvae of the ALB. The larvae were permissive to all four species but mortality was higher and production of infective juveniles (IJ) was greater for S. carpocapsae and H. marelatus. The lethal dosage of H. marelatus was determined to be 19 IJ's for second and third instar larvae, and 347 IJ's for fourth and fifth instar larvae. H. marelatus infective juveniles, applied via sponges to oviposition sites on cut logs, located and killed host larvae within 30 cm galleries, and reproduced successfully in several of the larvae. H. marelatus killed fifth instar host larvae in 2-6d [@ 500 (n=3) and 2000 IJ/larva (n=5)]. H. marelatus reproduction within fifth instar host larval cadavers ranged from 158-321 x 103 nematodes per cadaver. While the S. carpocapsae isolate is currently being evaluated in ID-50 and LD-50 studies and data have not as yet been quantitatively analyzed, it appears that S. carpocapsae kills its hosts somewhat faster than for H. marelatus. However, it also appears that S. carpocapsae reproduction within the dead hosts may be lower than for H. marelatus. Results of the initial studies have been published (Solter et.al. 2001).
Bacillus thuringiensis (D'Amico). Bacillus thuringiensis (Bt) was evaluated as a microbial insecticide against larval and adult ALB. Studies in which existing commercial Bt products ("whole" Bt requiring activation) were incorporated into diet and fed to larvae and adult ALB, showed that they were not effective against either ALB life stage. Voltage clamp assays resulted in the identification of several Cry toxins that were effective against ALB larval midgut in vitro, especially Cry 1B. Assay of available Cry 1B showed that it is not effective against ALB larvae in vivo. It was noted that midgut environment in vivo may not be suitable for Bt MOA, which may cause a discrepancy between in vitro work and bioassays. Brush Border Cell Assays (conducted by D. Dean, OSU) provided positive results that led to preparation of Cry toxin for use against adult ALB. This preparation, as well as new commercial products will be evaluated against adult ALB in 2002.
Microsporidia (Bauer). Working with Deborah Miller and Houping Liu, a new microsporidium isolate from ALB larvae was collected in Henan Province, China (December 2001), but it is as yet unidentified. Infection prevalence of larval samples was ca. 2% (n=97).
Predators and Parasitoids (Smith et.al.; Herard et.al.; Bauer et.al.). A number of natural enemies of the Cerambycidae have been reported worldwide, including predators belonging to the Cucujidae, Ostomidae, Cleridae, Colydiidae, and Elateridae beetles; Asilidae, Xylophagidae, and Rhagionidae flies; Phymatidae and Reduviidae bugs; and predaceous thrips and carpenter ants, as well as parasitoids belonging to the Braconidae, Ichneumonidae, Bethylidae, Encyrtidae, Eulophidae, Gasteruptiidae, Pteromalidae, Eupelmidae, and Eurytomidae, wasps; and Tachinidae and Sarcophagidae flies (Linsley 1961). As such, identification of effective parasitoids for use in eradication and/or management of ALB has been underway, both within the country of origin (China in particular), as well as outside the country of origin (including Europe and North America). Two approaches are being utilized in these efforts: (1) evaluation of natural enemies known to attack ALB in China; and (2) evaluation of possible new associations between ALB and natural enemies of other cerambycids in China, Europe and North America. However, in order to focus efforts on those natural enemy species with a greater probability of providing biological control of ALB, selection and prioritization of these natural enemies are based largely upon the relatedness of their respective longhorned beetle hosts to ALB at three levels: (1) phylogenetic relatedness; (2) ecological relatedness (e.g. climate; habitat; host tree species and condition); and (3) behavioral relatedness (e.g. larval feeding behavior) (Smith 1999). Certain aspects of each of these likely play a major role in determining the potential efficacy of a given natural enemy to control ALB, as well as other Anoplophora species.
China: In China, parasitoids have been identified that are known to attack longhorned beetles that share a common host tree with ALB, as well as those known to attack ALB and/or other Anoplophora species. Among the first group are several egg parasitoids, including: the encyrtids Oophagus batocerae and Zaommoencyrtus brachytarsus, parasitoids of Batocera horsfieldi, and Austroencyrtus ceresii, a parasitoid of Ceresium sinicum; and the eulophid Aprostocetus prolixus, a parasitoid of Apriona germarii. Also among the first group are larval parasitoids, including: the tachinid Bullaea sp., the bethylid Scleroderma guani, a parasitoid of Saperda populnea, Semenotus bifasciatus and Semenotus sinoauster; the braconids, Ontsira palliates, a parasitoid of Semenotus bifasciatus and Semenotus sinoauster, and Zombrus bicolor and Zombrus sjoestedti, larval parasitoids of cerambycid spp.; and the ichneumonids Xylophrurus coreensis, Schreineria sp and Megarhyssa sp., larval parasitoids of cerambycid spp. Among the second group is the egg parasitoid Aprostocetus fukutai (Eulophidae), which parasitizes both Anoplophora chinensis and A. germarii (Liao et al, 1987; Wang and Zhao, 1988). However, no egg parasitoids have as yet been reported from ALB or A. nobilis (Yan and Qin, 1992; Zhou, 1992). Also among this second group are several larval parasitoids, including the braconids Ontsira sp. parasitizing A. chinensis larvae, and Ontsira anoplophorae sp. nov., parasitizing Anoplophora malasiaca on citrus; as well as the Colydiidae beetle Dastarcus longulus, a larval-pupal parasitoid of ALB, A. nobilis, B. horsfieldi, A. germarii, Monochamus alternatus, and Trirachys orientalis (Qin and Gao, 1988).To date, investigations by Smith et. al. have found no egg parasitoids of ALB. Therefore, their efforts have focused in large part on two of the species mentioned above, S. guani and D. longulus. Primary objectives have been to evaluate their relative efficacy to parasitize ALB, and to develop mass rearing technology. Results from studies of S. guani, to date, have shown that S. guani is an idiobiont ectoparasitoid, and females first paralyze their host by stinging, which immobilizes the host, and then lay eggs on the host body. Larvae are gregarious while developing on their host. After hosts are consumed, mature wasp larvae spin cocoons and pupate. An average of 45 adult S. guani emerged from a single mature host larva of Saperda populnea. In nature, S. guani was found parasitizing 41.9 - 92.3% of S. populnea larvae in poplar stands in many areas. Parental wasps remain with their young until they have completed their development and emerged as adult wasps. Should their eggs or larvae become separated from the host, parental wasps have been observed to return them to the host. Most female wasps are apterous, and S. guani usually parasitizes longhorned beetle species whose larvae are small, ca. 15 mm in length. Therefore, S. guani would be used to specifically target ALB 1st to 3rd instar larvae. Results from studies of D. longulus, to date, have shown that it is an ectoparasitoid, with females laying eggs in frass and sawdust in host gallery or on the host gallery wall. First instar larvae possess thoracic legs and crawl in search of a host. Upon finding an acceptable host, the larvae lose their thoracic legs and attach to the body of its host for feeding. It feeds singly or gregariously on its host, and as many as 30 individuals of this parasitoid are capable of successfully completing their development on a single ALB larva or pupa, which usually kills the ALB within 10 days. In many areas, parasitization rates of ALB by D. longulus has found to reach between 50-70%, and in locations where D. longulus is established in relatively high numbers, ALB is said to be under natural control. Investigations focused on development of mass rearing technologies for both of these species are in progress and are promising. Given their respective optimal preferences for different sized larvae, as well as the wingless nature of S. guani, inundative releases of these two species, in tandem, appears to offer a possible complementary approach to the existing strategies in the ALB eradication program. However, prior to releases, non-target studies are planned and will be conducted at BIIR. Results of the initial studies have been published (Smith et.al. 1999; Yang and Smith 2000, 2001)
Europe: Herard et. al. recently initiated investigations of potential natural enemies of ALB in Europe, with an initial emphasis on studies of Saperda populnea (L.) and Saperda carcharias (L.). These two species were selected because they share common traits with ALB: (1) both are Lamiinae; (2) both attack trembling aspens, poplars, and willows, trees that are among the preferred hosts of ALB in China; and (3) both attack healthy trees. While no egg parasitoids have been found in France to date, the eulophid Euderus caudatus has been reported as an egg parasitoid of S. populnea and S. carcharias. Two early larval parasitoids have been found thus far: a tachinid (not yet been identified) from France (southern and eastern) and Finland, and the eulophid Euderus albitarsis from southern Finland, where it was found parasitizing 1st instar S. populnea larvae. Two parasitoids whose adults emerged from full-grown larvae of S. populnea were found in 2001: the tachinid Billaea irrorata and the ichneumonid Dolichomitus populneus, previously mentioned from S. populnea and S. carcharias. Although B. irrorata emerges fairly late during its host development, its ability to attack very early larval instars will be elucidated. Rate of parasitism by each species in the various sites has not as yet been determined.
In addition, the following predatory Diptera larvae were found by dissection of branches in S. populnea galleries: Odinia xanthocera (Diptera, Odiniidae), Lasiambia baliola (Diptera, Chloropidae), and Thaumatomyia elongatula (Diptera, Chloropidae). While no braconids have been found to date, 4 species are known parasitoids of S. populnea and 1 species is known to parasitize S. carcharias. Among tachinids, two other species are known (one from S. populnea and one from S. carcharias). Among Ichneumonids, 22 other species are known from S. populnea, and 11 other species are known from S. carcharias. Consequently, it appears that the biocomplex of enemies of these 2 cerambycids in Europe constitutes a great reservoir of species that can be tested against Anoplophora spp.
In concert with identifying and selecting candidate natural enemies for evaluation against ALB, development of laboratory rearing techniques for the cerambycid species, specifically on live plant material, was initiated. Studies that are planned for 2002 include: (1) continue exploration and develop an inventory of early stage parasitoids of S. populnea and S. carcharias across Europe; (2) finalize S. populnea and S. carcharias rearing techniques using rooted cuttings; (3) implement ALB rearing techniques in 5-10 cm diameter rooted cuttings; (4) test Saperda spp. parasitoids on ALB, in quarantine at Montpellier; and (5) survey ALB and Anoplophora chinensis populations in sites where these 2 species were accidentally introduced in Europe, for possible occurrence of parasitism by local species. The anticipated product(s) from these studies are parasitoids of the Western Palearctic region cerambycids that show promise as efficacious biological control agents against early stages of ALB, and which can be used in the Nearctic region without significant non-target effects on North American ecosystems.
North America: Smith and Fuester, and Bauer et.al. recently initiated investigations of potential natural enemies of ALB in North America. Both groups, to date, have found a dipteran parasitoid associated with ALB-infested trees (Bauer et.al. from Chicago, 2001) (Smith et.al. in Norway maple trees from New York, 1998), which has not yet been identified. In addition, however, to searching for natural enemies associated with ALB-infested trees in New York and Chicago, efforts to evaluated parasitoids of selected North American cerambycids are underway. To reiterate, in an effort to identify North American natural enemies that are most likely to adapt to ALB, Smith (1999) proposed that priority should be given to those natural enemy species whose cerambycid hosts are most similar/related to ALB (phylogenetically, ecologically and behaviorally).
References Cited:
Cavey, Joseph F. 1998. Solid wood packing material from China: Initial pest risk assessment on certain wood boring beetles known to be associated with cargo shipments: Asian Longhorned Beetle (Anoplophora glabripennis), Ceresium, Monochamus and Hesperophanes. USDA-APHIS.
Liao Dingxi, Li Xueliu, Pang Xiongfei and Chen Tailu. 1987. Hymenoptera: Chalcidoidea(1). Economic Insect Fauna of China Fasc. 34. Science Press, Beijing, 241 pp., 24 plates.
Linsley, E. G. 1961. The Cerambycidae of North America, Part I. Introduction. Univ. Calif. Publ. Entomol. 18: 1-135.
Nowak, D. J., J. E. Pasek, R. A. Sequeira, D. E. Crane, and V. C. Mastro. 2001. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J. Econ. Entomol. 94 (1): 116-122.
Qin Xixiang and Gao Ruitong. 1988. Dastarcus longulus biological characteristics and its application. Kunchongzhishi 25(2):109-112.
Smith, Michael T. 1999. The potential for biological control of Asian Longhorned Beetle in the U.S. Midwest Biological Control News 6: 1-7.
Smith, Michael T., Ruitong Gao, Yang Zhong-qi and Baode Wang. 1999. Anoplophora glabripennis (Motschulsky): Field behavior and natural enemies in China, pp. 60-61. In: S.L.C. Fosbroke and K.W. Gottschalk (eds.), Proceedings U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and other Invasive Species. Annapolis, MD.
Solter, L.F., Keena, M., Cate, J.R., McManus, M.L. and Hanks, L.M. 2001. Infectivity of four species of nematodes (Rhabditoidea: Steinernematidae, Heterorhabditidae) to the Asian longhorn beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biocontrol Sci. and Technol. 11, 547-552.
US Forest Service. 2001. USFS report on the Asian Longhorned Beetle. http://www.na.fs.fed. us/spfo/alb/data/nyinfest.htm and http://www.na.fs.fed.us/spfo/alb/data/ilinfest.htm.
Wang Yong-jun & Zhao Zi-chen. 1988. A preliminary study on Aprostocetus sp. parasitizing on Apriona germarii (Hope). Kunchongzhishi 25(6): 347?350.
Xiao, G. 1992. Forest insects of China. China Forestry Publishing House. 455.
Yan, J. J. 1985. Research on distribution of basicostal white-spotted longicorn in east China. Journal of the North- Eastern Forestry College 13: 62-69.
Yan Junjie and QinXixiang. 1992. Anoplophora glabripennis (Motsch.). In: Xiao Gangrou (ed.), Chinese Forest Insects, pp. 455?457.
Yang, Zhong-qi, and Michael T. Smith. 2000. Insect natural enemies and their potential for biocontrol of Asian Longhorned Beetle, 72-73. In: S.L.C. Fosbroke and K.W. Gottschalk (eds.), Proceedings U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and other Invasive Species. Annapolis, MD.
Yang, Zhong-qi, and Michael T. Smith. 2001. Investigations of natural enemies for biocontrol of Anoplophora glabripennis (Motsch.), 139-141. In: S.L.C. Fosbroke and K.W. Gottschalk (eds.), Proceedings U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and other Invasive Species. Annapolis, MD.
Yang, X., Zhou, F. Wang, and M. Cui. 1995. A study on the feeding habits of the larvae of two species of longicorn (Anoplophora) to different tree species. Journal of Northwest Forestry College 10: 1-6.
Zhou Jiaxi. 1992. Anoplophora nobilis Ganglbauer. In: Xiao Gangrou (ed.), Chinese Forest Insects, pp. 458-459.
Infectivity of Rhabditoid Nematodes to the Asian Longhorn Beetle
Leellen F. Solter1, Melody Keena2, James R. Cate, Michael L. McManus2
M. Catherine Higgs1, and Lawrence M. Hanks4The Asian longhorn beetle, Anoplophora glabripennis (Motchulsky), recently introduced to the United States, is a pest of many species of trees in the urban and woodland environment, as well as a threat to the sugar maple industry (USDA/Forest Service Publ. NE-INF-140-00, 1999). The boring activity of larvae through in the cambium and through heartwood causes extreme stress to the host trees which, added to pre-existing stress conditions extant in the urban environment, frequently leads to their death. Efforts are underway to develop environmentally safe, biologically-based methods to control A. glabripennis that do not entail destruction of the entire infested tree. Rhabditoid nematodes have been used successfully as microbial insecticides in control programs for other cryptic pests. The restricted movement of A. glabripennis larvae and moist protected environment within their galleries suggest that nematodes, particularly searching species, may have some potential as a control method. Several studies in China found that entomopathogenic nematodes reduced the number of new A. glabripennis emergence holes when they were introduced into trees through existing emergence holes.
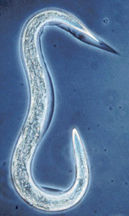
Steinernema carpocapsae nematode
ALB larva killed by nematodesFour species of rhabditoid nematodes produced by Integrated BioControl Systems, Inc. were tested for their ability to kill and reproduce in A. glabripennis larvae: Steinernema carpocapsae (Weiser) 1955, Sal strain; Heterorhabiditis bacteriophora Poinar 1976, Lewiston strain; H. indica Poinar, Karunakar and David 1992, HOM-1 strain; and H. marelatus Liu & Berry 1996, IN strain. The A. glabripennis larvae were permissive to all four nematode species, however, host mortality, and survival and reproduction of nematodes were highest for H. marelatus and S. carpocapsae. Bioassays with H. marelatus estimated that the lethal dosage (LD50) was approximately 19 infective juvenile nematodes for second and third instars and 347 nematodes for fourth and fifth instars. H. marelatus infective juveniles on moistened sponges were stapled to oviposition sites on cut logs and were able to locate and invade host larvae within 30 cm galleries.
1Illinois Natural History Survey, Center for Economic Entomology,
607 E. Peabody Dr., Champaign, IL, 618202 USDA Forest Service, Northeastern Center for Forest Health Research
51 Millpond Rd. Hamden, CT 065143Integrated BioControl Systems, Inc., Aurora, IN 47001
4University of Illinois, Dept. of Entomology, 505 Gregory St., Urbana, IL 61801
Investigations of Natural Enemies for Biocontrol of Anoplophora glabripennis (Motsch.)
Yang, Zhong-qi1 and Michael T. Smith2The Asian Longhorned Beetle (ALB), Anoplophora glabripennis Motsch., is a recent invader to the U.S. from China, with known infestations in New York (New York City and Long Island) and Illinois (Chicago). Although ALB is currently limited in distribution within the U.S., its potential for spread into other North American landscapes at risk is alarming and demands greater attention. The only method used to control ALB in China and the U.S. at present is through the removal of infested trees, and the current emphasis of much research is directed towards eradication. However, in the event that eradication is not successful, either in the known infestations in New York and Chicago, or in as yet undetected infestations elsewhere, alternative pest management approaches must be developed. In addition, even with complete eradication, new introductions are likely to occur as a result of the challenges of interception of infested cargo. For example, current interception efforts are focused on cargo that enters the U.S. directly from China, while cargo from other countries, which in fact originated in China, is extremely difficult to track and intercept. Collectively therefore, survey, evaluation and mass rearing of natural enemies of ALB in China, as well as similar investigations of natural enemies of related cerambycids in the U.S., have been initiated. The objectives of this research include the identification of highly effective and host specific, self-propagating natural enemies of ALB that possess a high potential for establishment (classical biocontrol, which tends to be the most cost effective approach for biological control), as well as those natural enemies that could be easily reared and utilized in inundative-release programs. In addition, since a long-term management goal may more realistically be to slow the ecological damage of this invader, native natural enemies (to the US) that adapt to ALB and/or its host trees may be of particular interest.
Compared with other longhorned beetles, relatively few natural enemies of ALB have thus far been identified. Prior to the initiation of these studies, no egg parasitoids of ALB had been reported. On the other hand, larval parasitoids had been reported, including Dastarcus longulus Sharp (Coleoptera: Colydiidae), Scleroderma guani Xiao et Wu (Hymenoptera: Bethylidae), Bullaea sp (Diptera: Tachinidae), and Megarhyssasp. (Hymenoptera: Ichneumonidae). Likewise, pupal parasitoids had also been reported, including D. longulus, S. guani, and Aprostocetus sp. (Hymenoptera: Eulophidae). Among these, D. longulus and S. guani, appeared to be the most important among these natural enemies of ALB since they were reported to be larval-pupal parasitoids.
D. longulus, in many areas, has been reported to have parasitization rates of 50-70%. Female D. longulus lay eggs in frass and sawdust in host gallery or on the host gallery wall. First instar larvae possess thoracic legs and crawl about in search of a host. Upon finding an acceptable host, the larvae lose their thoracic legs and attach to the body of its host for feeding. It is an ectoparasite, feeding singly or gregariously on its host (1-27 individuals per host), but in all cases the host is killed. D. longulus is considered to have the highest potential for use in biological control of ALB.
S. guani usually parasitizes longhorned beetle species whose larvae are small, ca. 15 mm in length. It is an idiobiont ectoparasitoid. Female wasps first paralyze their host by stinging, which immobilizes the host, and then lay eggs on the host body. Larvae are gregarious while developing on their host. After hosts are consumed, mature wasp larvae spin cocoons and pupate. Parental wasps remain with their young until they have completed their development and emerged as adult wasps. Should their eggs or larvae become separated from the host, parental wasps have been observed to return them to the host. Most female wasps are apterous. S. guani can be mass reared for biocontrol. Therefore, S. guani has great potential for use in the biological control of ALB larvae, specifically 1st to 3rd instars.1999
Surveys for natural enemies were conducted in Shaanxi, Shanxi, Hebei, Xinjiang, NeiMongol (Inner Mongolia), Heilongjiang and Shandong Provinces. As such, while over 560 ALB eggs were collected, no ALB egg parasitoids were recovered. However, four ALB larval parasitoids were found, including: D. longulus, S. guani, Zombrus sjostedti (Fahringer)(Hymenoptera: Braconidae), and Megarhyssa sp.
Initial studies of D. longulus resulted in parasitization rates of 25-95% in Shaanxi Province. Furthermore, these studies showed that 1-18 individual D. longulus completed development on a single host larva, and resulted in 100% ALB larval mortality. Preliminary studies of S. guani showed that it could parasitize both ALB larva (3rd and 4th instar) and pupa. Preliminary studies of the biology and behavior of Aprostocetus prolixus LaSalle et Huang (Hymenoptera: Chalcidoidea: Eulophidae, Tetrastichinae), an egg parasitoid of Apriona germari (Hope)(Coleoptera: Cerambycidae), indicated that it may have potential as an ALB egg parasitoid, and thus studies were planned for 2000.2000
A total 1,256 ALB eggs were collected in Shaanxi, Hebei and Ningxia Provinces, but no egg parasitoids were again identified. However, an egg parasitoid of Batocera horsfieldi (Hope), another important longhorned beetle pest of popular in China, was collected, and appears to be a new species. Description of this species is in progress.
Studies of D. longulus were continued and showed that it overwinters as an adult in the crevice of old bark, as well as in the soil near ALB-infected tree. A total of 650 overwintering adults were collected during this survey. Results indicated that its life span may exceed 5 month, that it can be reared with artificial diet in 30 days. Finally, indications are that D. longulus population levels are lower in monocultural stands than in species rich stands. This corresponds with higher ALB population levels in monocultural stands than in species rich stands. Although studies are still in progress, results indicate that D. longulus may be selected as an effective biological control agent of mature ALB larvae.
Studies of the larval parasitoid, S. guani, resulted in the identification of an excellent substitute host for mass rearing S. guani. The substitute host is inexpensive and easily obtained. In addition, lab and fields experiments were conducted, and results confirmed that S. guani can control young ALB larvae. A parasitization rate of approximately 65% was obtained in lab studies, and field studies are still in progress.
Studies of A. prolixus, an egg parasitoid of A. germeri, indicated that this parasitoid does not diapause. While control temperature experiments showed that A. prolixus emergence could be adjusted to coincident with ALB oviposition, and that the wasp could parasitize 20-50% A. germari eggs, it did not parasitize ALB eggs. Additional studies are planned.Summary
BIIR is currently the only U.S. lab examining insect parasitoids, and several promising ALB-specific biological control agents have already identified. The research on rearing natural enemies is an important weapon for pest management and current results are encouraging. Collectively, these studies should contribute greatly to the development of an Integrated Pest Management Program for ALB in the U.S.
1Research Institute of Forest Ecology, Environment and Protection Chinese Academy of Forestry, Beijing, CHINA 1000912 USDA Agricultural Research Service, Beneficial Insects Introduction Research Lab 501 S. Chapel Street, Newark, DE 19713
Research Home