Research in the Waterman Group addresses
problems in synthesis, catalysis, materials, and energy
through the application of organometallic systems. These
efforts are directed at the discovery of new synthetic methods
in the main group, the preparation of novel materials, and
development of efficient or "green" syntheses through
catalysis.
I. BOND-FORMING CATALYSIS
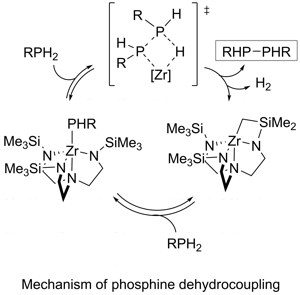
We have been investigating methods to catalytically form bonds between main group elements. In particular, we have been looking at generating bonds to phosphorus. There is a rich synthetic chemistry associated with phosphorus; however, methods to generate those bonds catalytically are sparse.
To meet this end, we are applying early transition-metal complexes in dehydrocoupling reactions. Dehydrocoupling catalysis effectively exchanges element-hydrogen bonds from two molecules to form an element-element bond with liberation of hydrogen. We have recently demonstrated that triamidoamine complexes of zirconium are effective catalysts for the dehydrocoupling of phosphines. Further, we found that the catalysis appears to rely on sigma-bond metathesis steps for P-P bond formation (right). Applying this knowledge, we have already demonstrated selective P-Si and P-Ge bond-forming catalysis.
Our main goal in developing these new methods is to use this kind of catalysis in the synthesis of novel materials that may exhibit unique properties.
II. CHEMICAL STORAGE OF HYDROGEN
Simple main-group molecules are being considered as method to storing hydrogen because of the inherent difficulties associated with pressurization, liquefaction, and physisorption. We are applying our knowledge of dehydrocoupling catalysis to develop an understanding of how hydrogen released from simple inorganic molecules such as amine-boranes with the broader goal of developing strategies to reversibly liberate hydrogen from these molecules.
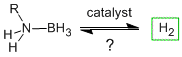
III. SYNTHESIS INVOLVING LOW-VALENT FRAGMENTS
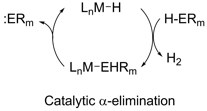
Another route we are exploring to forming element-element bonds is through the generation of low-valent fragments. Molecular precursors to low valent fragments are known for lighter elements such as carbon or nitrogen. This is not true for the heavier main-group elements. We have been investigating alpha-elimination, or the extrusion of a low-valent fragment from a transition-metal, as one possible route to these kind of species. Recently, we have discovered the first instance of catalytic alpha-arsinidene elimination.
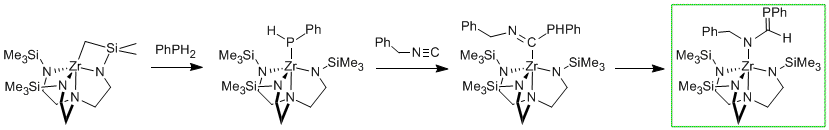
We are also interested in discovering innovative routes to accessing low-valent main-group fragments. A recent development in this area is our preparation of a phosphaalkene (molecule with a P=C bond) by insertion of an isocyanide into a zirconium-phosphorus bond. In this reaction, we take a commercially available phosphorus source, a primary phosphine, and effectively access a phosphinidene ("PR") fragment with perfect atom economy.
IV. CARBON DIOXIDE ACTIVATION
In collaboration with Rick Kemp at UNM/Sandia National Laboratories and Bill Geiger at UVM, we have been developing zinc and tin complexes to coordinate carbon dioxide and facilitate electrochemical reduction.

RESEARCH SUPPORT
Research in the Waterman Group is/has been generously supported by a number of agencies through grants and awards. 





I. BOND-FORMING CATALYSIS
We have been investigating methods to catalytically form bonds between main group elements. In particular, we have been looking at generating bonds to phosphorus. There is a rich synthetic chemistry associated with phosphorus; however, methods to generate those bonds catalytically are sparse.
To meet this end, we are applying early transition-metal complexes in dehydrocoupling reactions. Dehydrocoupling catalysis effectively exchanges element-hydrogen bonds from two molecules to form an element-element bond with liberation of hydrogen. We have recently demonstrated that triamidoamine complexes of zirconium are effective catalysts for the dehydrocoupling of phosphines. Further, we found that the catalysis appears to rely on sigma-bond metathesis steps for P-P bond formation (right). Applying this knowledge, we have already demonstrated selective P-Si and P-Ge bond-forming catalysis.
Our main goal in developing these new methods is to use this kind of catalysis in the synthesis of novel materials that may exhibit unique properties.
II. CHEMICAL STORAGE OF HYDROGEN
Simple main-group molecules are being considered as method to storing hydrogen because of the inherent difficulties associated with pressurization, liquefaction, and physisorption. We are applying our knowledge of dehydrocoupling catalysis to develop an understanding of how hydrogen released from simple inorganic molecules such as amine-boranes with the broader goal of developing strategies to reversibly liberate hydrogen from these molecules.
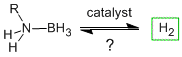
III. SYNTHESIS INVOLVING LOW-VALENT FRAGMENTS
Another route we are exploring to forming element-element bonds is through the generation of low-valent fragments. Molecular precursors to low valent fragments are known for lighter elements such as carbon or nitrogen. This is not true for the heavier main-group elements. We have been investigating alpha-elimination, or the extrusion of a low-valent fragment from a transition-metal, as one possible route to these kind of species. Recently, we have discovered the first instance of catalytic alpha-arsinidene elimination.
We are also interested in discovering innovative routes to accessing low-valent main-group fragments. A recent development in this area is our preparation of a phosphaalkene (molecule with a P=C bond) by insertion of an isocyanide into a zirconium-phosphorus bond. In this reaction, we take a commercially available phosphorus source, a primary phosphine, and effectively access a phosphinidene ("PR") fragment with perfect atom economy.
IV. CARBON DIOXIDE ACTIVATION
In collaboration with Rick Kemp at UNM/Sandia National Laboratories and Bill Geiger at UVM, we have been developing zinc and tin complexes to coordinate carbon dioxide and facilitate electrochemical reduction.

RESEARCH SUPPORT
Research in the Waterman Group is/has been generously supported by a number of agencies through grants and awards.
- U. S. National Science Foundtion (grant number CHE-0747612)
- Research Corporation for Science Advancement through a Cottrell Scholar Award to R. W.
- Alfred P. Solan Foundation through a Research Fellowship to R. W.
- subcontractor to LDRD award at Sandia National Laboratory
- American Chemical Society Petroleum Research Fund (grant number 466669-G3)
- Vermont Space Grant Consortium (Graduate Research Assistantship for Analese Maddox)
- Project SEED (summer funding for high school students)
- Univerity of Vermont (start-up funds)






Last modified March 29, 2013 7:55 AM
| Research in the Waterman Group addresses problems in synthesis, catalysis, materials, and energy through the application of organometallic systems. These efforts are directed at the discovery of new synthetic methods in the main group, the preparation of novel materials, and development of efficient or "green" syntheses through catalysis. |
| I. BOND-FORMING CATALYSIS |
| We have been investigating
methods to catalytically form bonds between main
group elements. In particular, we have been
looking at generating bonds to phosphorus. There
is a rich synthetic chemistry associated with
phosphorus; however, methods to generate those
bonds catalytically are sparse. To meet this end, we are applying early transition-metal complexes in dehydrocoupling reactions. Dehydrocoupling catalysis effectively exchanges element-hydrogen bonds from two molecules to form an element-element bond with liberation of hydrogen. We have recently demonstrated that triamidoamine complexes of zirconium are effective catalysts for the dehydrocoupling of phosphines. Further, we found that the catalysis appears to rely on sigma-bond metathesis steps for P-P bond formation (right). Applying this knowledge, we have already demonstrated selective P-Si and P-Ge bond-forming catalysis. Our main goal in developing these new methods is to use this kind of catalysis in the synthesis of novel materials that may exhibit unique properties. |
|
| II. CHEMICAL STORAGE OF HYDROGEN |
| Simple main-group molecules are being considered as method to storing hydrogen because of the inherent difficulties associated with pressurization, liquefaction, and physisorption. We are applying our knowledge of dehydrocoupling catalysis to develop an understanding of how hydrogen released from simple inorganic molecules such as amine-boranes with the broader goal of developing strategies to reversibly liberate hydrogen from these molecules. | 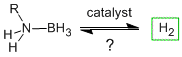 |
| III. SYNTHESIS INVOLVING LOW-VALENT FRAGMENTS |
| Another route we are exploring to forming element-element bonds is through the generation of low-valent fragments. Molecular precursors to low valent fragments are known for lighter elements such as carbon or nitrogen. This is not true for the heavier main-group elements. We have been investigating alpha-elimination, or the extrusion of a low-valent fragment from a transition-metal, as one possible route to these kind of species. Recently, we have discovered the first instance of catalytic alpha-arsinidene elimination. | |
| We are also interested in discovering innovative routes to accessing low-valent main-group fragments. A recent development in this area is our preparation of a phosphaalkene (molecule with a P=C bond) by insertion of an isocyanide into a zirconium-phosphorus bond. In this reaction, we take a commercially available phosphorus source, a primary phosphine, and effectively access a phosphinidene ("PR") fragment with perfect atom economy. |
 |
| IV. CARBON DIOXIDE ACTIVATION |
| In collaboration with Rick Kemp at UNM/Sandia National Laboratories and Bill Geiger at UVM, we have been developing zinc and tin complexes to coordinate carbon dioxide and facilitate electrochemical reduction. |  |
| RESEARCH SUPPORT |
Research in the Waterman Group
is/has been generously supported by a number of
agencies through grants and awards.
|
 |
 |

|
 |

|

|